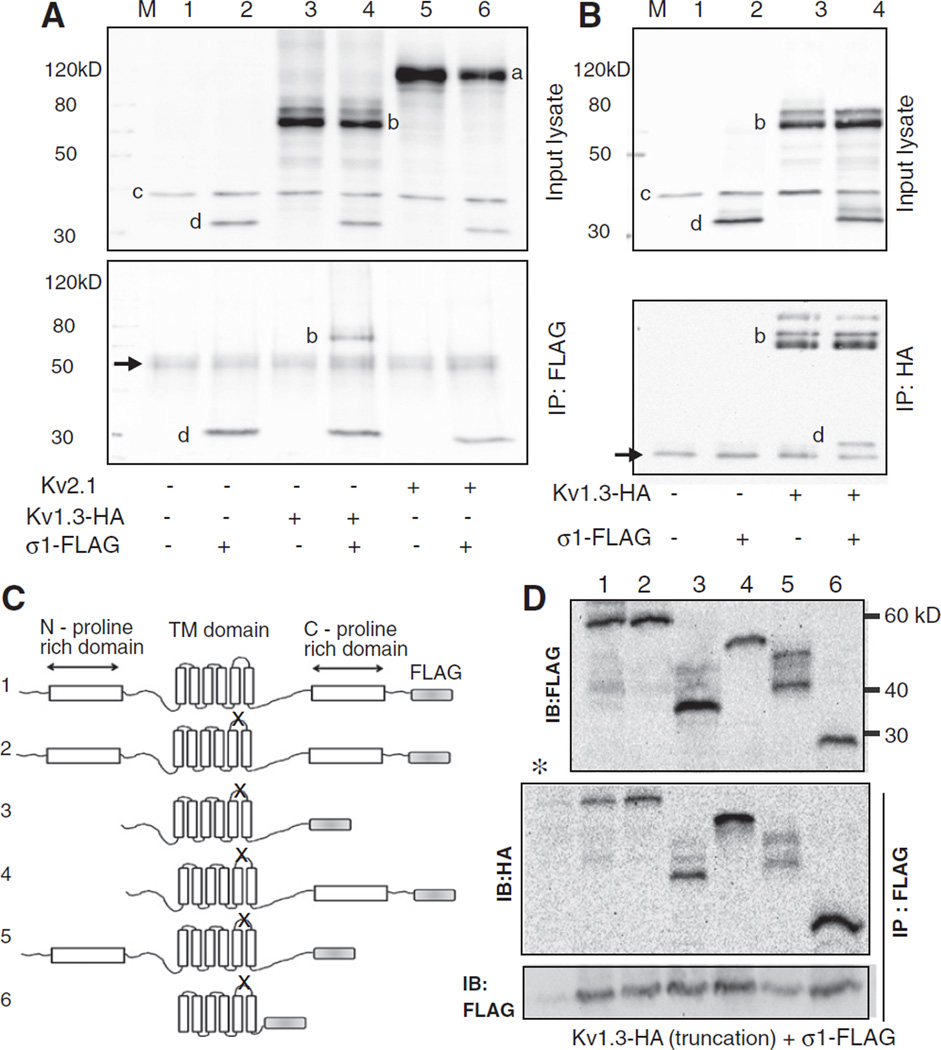
Fig. 4.
Co-immunoprecipitation study of protein–protein interaction between Kv1.3 and Sigma1R. A. Lysates from HEK 293 cells transfected with HA-tagged Kv1.3 and FLAG-tagged Sigma1R and that immunoprecipitated with anti-FLAG antibody were blotted with a cocktail of mouse anti-Kv2.1 (1:1000), -HA (1:1000, for Kv1.3), -GAPDH (1:100,000), and -FLAG (1:2000, for Sigma 1R) antibodies. The top panel is the input lysate and the bottom after immunoprecipitation. Note the presumed Kv2.1 (a), Kv1.3-HA(b), GAPDH(c), and Sigma 1R-FLAG(d) immunoreactive bands (top) and the IgG heavy chain band (arrow, bottom). Both panels are blots of the entire membrane. B. A reverse co-IP experiment where the input lysates (top) were immunoprecipitated with the anti-HA antibody and probed with a mixture of antibodies as above (bottom).Arrow (bottom panel) is the IgG light chain band. C. Adiagram of various truncated non-conducting (denoted by X in the P-loop) Kv1.3-HA and wild type full length Sigma1R-FLAG. Characteristic structural motifs shown include the N- and C-proline rich domains and the six transmembrane domains. The channel constructs were: 1.wild type full length Kv1.3, 2. non-conducting (P loop GYG to AYA, n1560–1568) full length, mutant, 3.N- and C-proline rich domain deleted non-conducting mutant (ATG at n633–635 and TGA at n1845–1847), 4. N-proline rich domain deleted non-conducting mutant (ATG at n633–635), 5. C-proline rich domain deleted non-conducting mutant (TGA at n1845–1847), and 6. core domain non-conducting mutant (ATG at n906–908 and TGA at n1671–1673). All nucleotide numbers are as denoted on NCBI accession number NM_002232. The expected molecular mass of the constructs are 55.5, 55.5, 45.8, 49.5, 48, and 27.7 kDa for constructs 1–6, respectively. D. Anti-FLAG or anti-HA immunoblots of lysates from cells co-transfected with the various truncated Kv1.3-HAconstructs and Sigma1R-FLAG (top). Kv1.3 proteins that co-immunoprecipitated with Sigma1R are denoted in the IP: Flag IB:HAblot (middle). (*) denotes a control lane identical to lane 1 lysate but immunoprecipitated with an unrelated antibody. Control (IP: FLAG, IB: FLAG) (bottom) confirmed successful immunoprecipitation in all lanes except the negative control * lane. The images are over-exposed to allow detection of the faint protein degradation or post-translationally modified mobility-shifted species. All immunoblots were repeated a minimum of 3 times with consistent results.