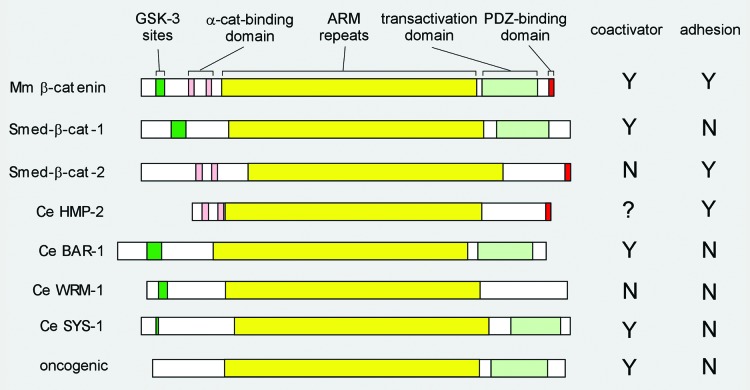
Figure 1. β-catenin domain structure. Schematic representation of the indicated β-catenin proteins with functional domains referred to in the text highlighted in color: central ARM repeat domain in yellow; coactivator-associated domains in shades of green: GSK-3 phosphorylation sites (dark green) and C-terminal transactivation domain (light green); cell-cell adhesion-associated domains in shades of red: N-terminal α-catenin-binding domain (pink) and C-terminal PDZ-binding domain (red). The bifunctional mouse (Mm, top) β-catenin is used here to represent the canonical single β-catenins in human, Xenopus, and flies. The two monofunctional planarian (Smed) proteins are above the four C. elegans (Ce) proteins, followed by the oncogenic form of β-catenin (bottom). GSK-3 phosphorylation motif: typically DpSGΦXpS/T, where Φ is a hydrophobic amino acid (V, I, L, M, F, W, or C). This motif is often coupled with additional S/TxxxS/T motifs. Mouse β-catenin, planaria β-catenin-1, and Ce BAR-1 contain this motif near their N-termini. The three other Ce proteins lack this motif, but do have 2–3 possible GSK-3 sites (simple (S)/(T)xxx(S)/(T) near their N-termini. α-catenin-binding and the C-terminal PDZ-binding domains: Both planaria β-catenin-2 and C. elegans HMP-2 bind α-catenin (experimentally tested) through a domain similar to the α-catenin-binding domain in mouse (TRAQRVRAAMFPE ..21.. VQRLAEPSQMLK). All three proteins also have a PDZ-binding domain at their very C-terminus (mouse: WFDTDL[stop]). The other three C. elegans β-catenins do not exhibit sequence related to either motif and have been tested negative in α-catenin binding assays. C-terminal transactivating domain: Transcription activation domain was normally tested in a TOPFLASH reporter assay. Only BAR-1 and SYS-1 activate a TOPFLASH reporter in tissue culture cells. However, this assay does not accurately measure the transactivation domain of β-catenin as this assay requires binding of β-catenin to the N-terminal domain of the TCF/POP-1 protein. Natarajan (2001) reported that both BAR-1 and HMP-2 can activate transcription in a yeast 1-hybrid assay, but BAR-1 did so significantly better than HMP-2. WRM-1 was shown to have a very weak, slightly above background activation capability in such an assay.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.