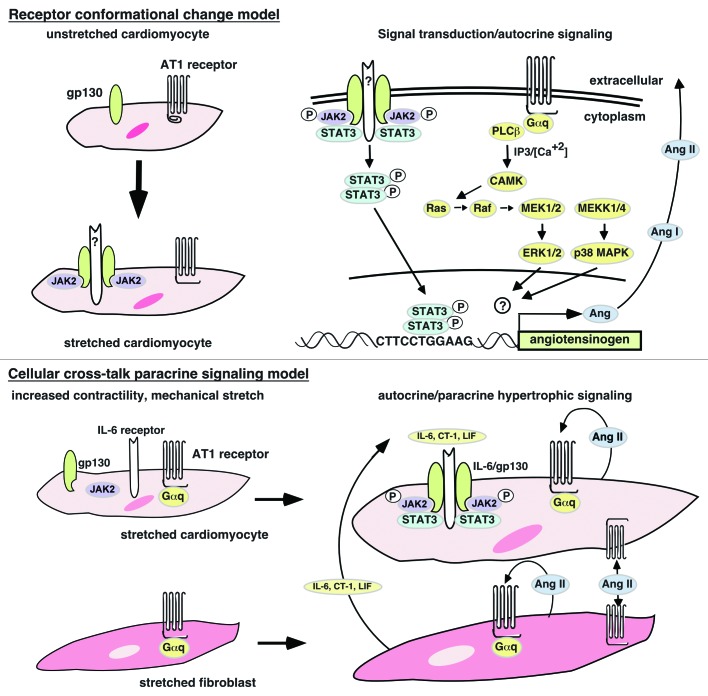
Figure 1. Models of mechanical stretch activation of JAK kinases and STAT proteins. Conformational model. Mechanical stretch is postulated to induce conformational changes in the gp130 and AT1 receptors that expose binding sites for JAK2 kinases and Gαq proteins, respectively. It is unclear how or if gp130 dimerizes in order to allow JAK2 kinase cross-phosphorylation. The AT1 receptor acting as a stretch receptor will activate the RAS/MEK/ERK/p38 pathway leading to upregulation of the angiotensinogen gene. The transcription factor activated by p38 or ERK1/2 has not been identified. Paracrine model. Mechanical stretch initiates signal transducer association with receptors as in conformational model. Continued signaling after mechanical stretch exhaustion is performed by Ang II produced in response to stretch-activated AT1 receptors to give autocrine signaling as well as paracrine signaling. Ang II-activated cardiac fibroblasts produce IL-6 family cytokines that activate the canonical JAK-STAT pathway in cardiomyocytes via paracrine signaling thereby maintaining the post-mechanical stretch hypertrophic state.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.