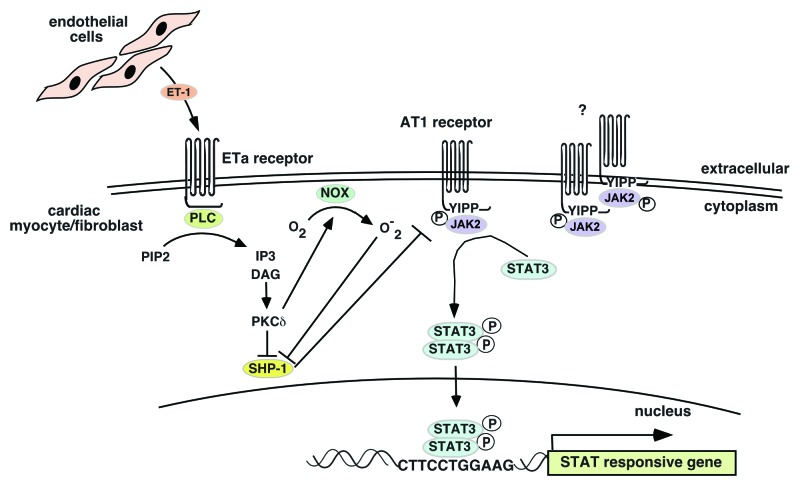
Figure 2. Endothelin-1 receptor crosstalk with Ang II receptor to potentiate JAK2 kinase activity. Endothelial cell-derived ET-1 activates the ETa receptor on cardiomyocytes or cardiac fibroblasts. Activated ETa signals to PKCδ, which mediates inhibition of the SHP-1 JAK2 kinase phosphatase to allow phosphorylated JAK2 kinases to remain phosphorylated and active. These active JAK2 kinases can phosphorylate STAT3 associated with either the AT1 or ETa receptor (which lacks a JAK2 binding site but has one for STAT3). How JAK2 kinases become phosphorylated to begin with is not clear. There is some evidence that the AT1 receptor can dimerize in which case the associated JAK2 kinases can cross-phosphorylate themselves.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.