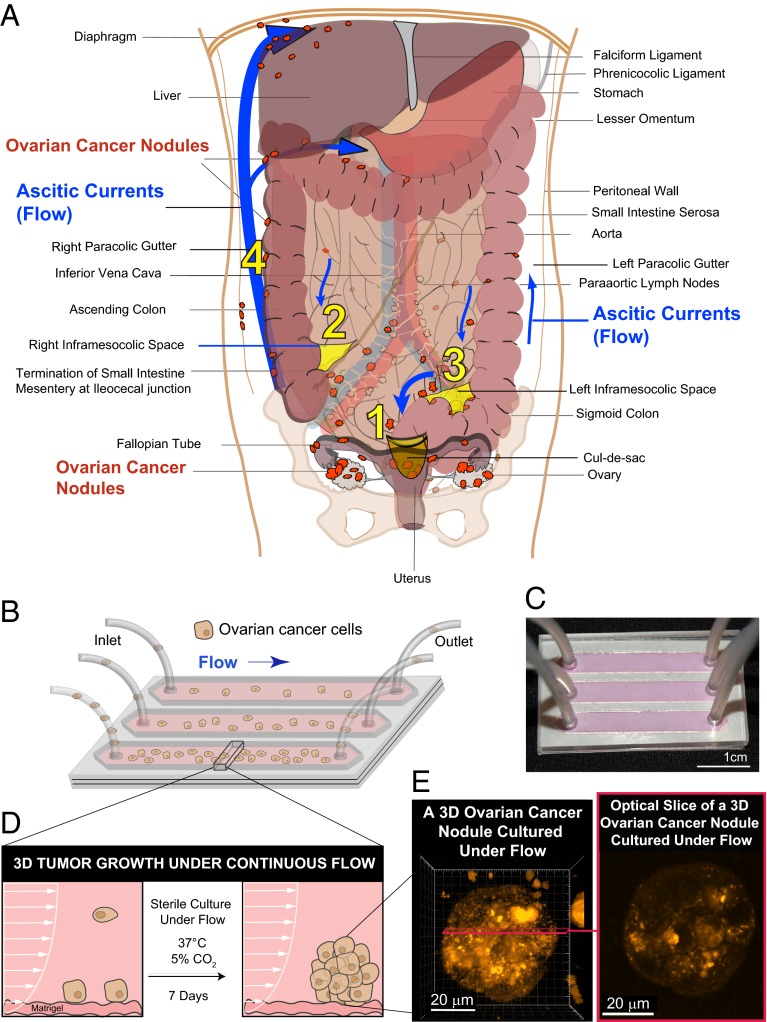
Fig. 1.
Modeling fluidic determinants of ovarian cancer dissemination and growth. Tumor cell dissemination and colonization of distant sites is influenced by a complex array of factors including the physical stresses that tumor cells encounter as they interact with stromal beds. (A) Ovarian cancer disseminates predominantly via movement of intraperitoneal fluid leading to a distinctive distribution pattern of tumor nodules (orange) involving four common abdomino-pelvic sites: (1) cul-de-sac (peritoneal fold between the rectum and the posterior wall of the uterus); (2) right infracolic space (the apex formed by the termination of the small intestine of the small bowel mesentery at ileocecal junction); (3) left infracolic space (superior site of sigmoid colon); (4) Right paracolic gutter (communication between the upper and lower abdomen defined by the ascending colon and peritoneal wall). This characteristic distribution is influenced by preferential pathways of ascitic flow (blue arrows) that are established by the hydrodynamics of intraperitoneal fluid motion in the abdomino-pelvic cavity. The direction and strength of these fluidic pathways are determined by physical influences including negative subdiaphragmatic pressure, gravity, and organ mobility as well as by recesses formed by key anatomical structures: (i) cul-de-sac, (ii) termination of small intestine mesentery, (s) sigmoid colon, (iv) falciform ligament, and (v) phrenicocolic ligament. In contrast to this flow-based dissemination, the absence of ascites leads to metastatic spread that is largely proximal to the primary tumor. (B) Schematic of a microfluidic chip used to study the effect of sustained flow on the growth and molecular features of 3D ovarian cancer nodules. (C) Photograph of a microfluidic chip used in the experiments. (D) A side view of a microfluidic chip designed to study the impact of flow on the attachment and growth of ovarian cancer cells to a stromal bed. (E) Ovarian cancer cells were cultured successfully under continuous flow for 7 d in the microfluidic chip and formed 3D micronodules as shown in a 3D rendering (Left) and an optical slice (Right) of a two-photon autofluorescence image.