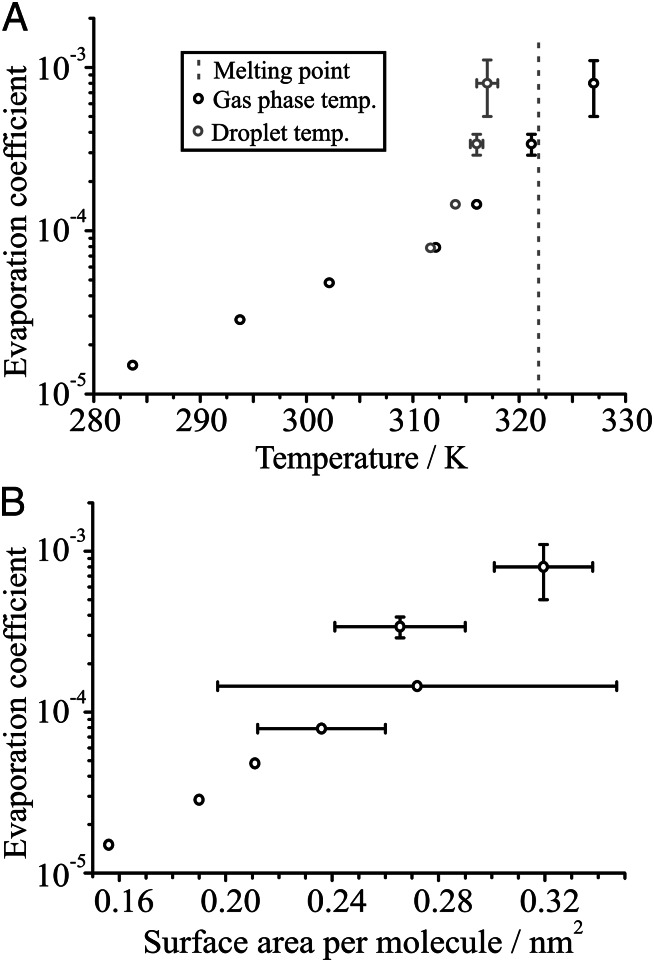
Fig. 3.
(A) The effect of varying the gas phase temperature (black points) on the evaporation coefficient for water droplets doped with 0.2 mM hexadecanol. The melting point of hexadecanol is indicated. Note that the droplet will be at a lower temperature than the gas phase due to evaporative cooling, an effect that becomes more important at high temperatures where the RH is lower and evaporation is more rapid. The droplet temperature, estimated from the mass flux equations, is plotted in gray, and the uncertainty in both axes is estimated based on the uncertainty in the RH. In cases in which no error bar is present, the point itself is equal to or much larger than the uncertainty in the measurement. (B) The dependence of the evaporation coefficient on the surface area per molecule of the monolayer, calculated for each of the temperatures presented in A. The uncertainty in surface area comes primarily from the initial size uncertainty, which increases with more the rapid evaporation at higher temperatures. The largest error bar is due to an anomaly in the observed formation of the monolayer, and the radius at the point of monolayer formation was less clear.