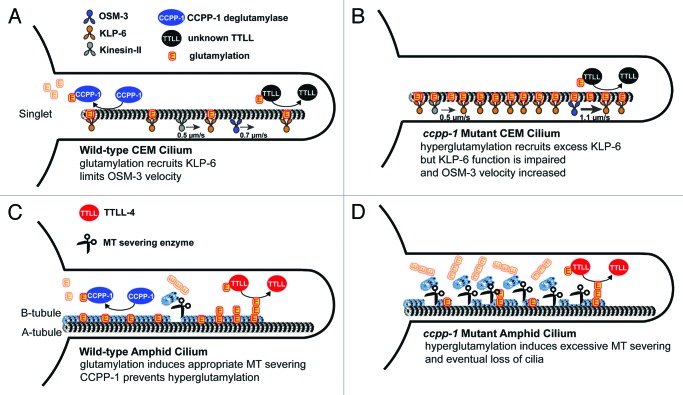
Figure 2. Model of cell-specific CCPP-1 deglutamylase function in CEM and amphid channel cilia. (A) Highly schematized diagram of a wild-type CEM cilium. MT singlets in CEM cilia are subject to glutamylation by an unknown enzyme, and deglutamylation by CCPP-1. Normally glutamylated MTs recruit the kinesin-3 KLP-6 and regulate velocity of the kinesin-2 OSM-3. (B) In ccpp-1 mutant hyperglutamylated state, excessive KLP-6 accumulates, perhaps in an inactive form, and OSM-3 moves abnormally fast as if unencumbered by KLP-6.17 The velocity of heterotrimeric kinesin-II is unaffected. (C) Diagram of a wild-type amphid cilium. The antagonistic activities of CCPP-1 and TTLL-4 control MT glutamylation, which in turn, regulates MT severing enzymes. (D) In the absence of CCPP-1, hyperglutamylation leads to excessive severing of B-tubules (although we depict longer glutamylation side-chains in amphids, the side-chain length is unknown in either cilium).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.