Figure 2.
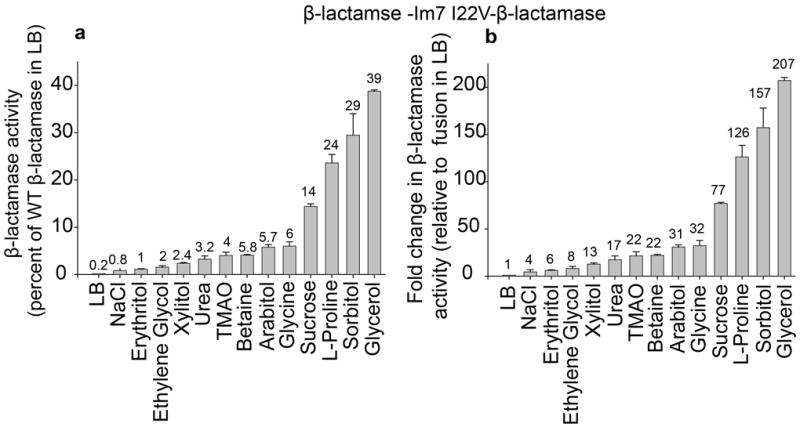
Influence of selected osmolytes on the β-lactamase activity of tripartite fusions containing the destabilized Im7 variant Im7 I22V. (a) β-lactamase activity (determined by measuring absorbance at 486 nm over time and adjusted to the cell OD of the cell extract) in whole cell extracts of Escherichia coli MG1655 ΔampCΔhsdR expressing the tripartite fusion β-lactamase -Im7 I22V-β-lactamase in the presence or absence of protein stabilizing agents in the cultivation medium. Enzymatic activity is expressed as % of the activity of cells expressing WT β-lactamase in LB (100%). (b) Fold change in β-lactamase activity in the presence of osmolytes in the cultivation medium compared to activity in LB of the same strain. 0.25 M NaCl was used as a negative control. The additives were all present at 0.5 M except for glycerol, which was used at 2 M, sorbitol, which was used at 1 M, urea, which was used at 200 mM and glycine, which was used at 0.1 M. Mean values ± standard deviations are shown for independent triplicate experiments.
