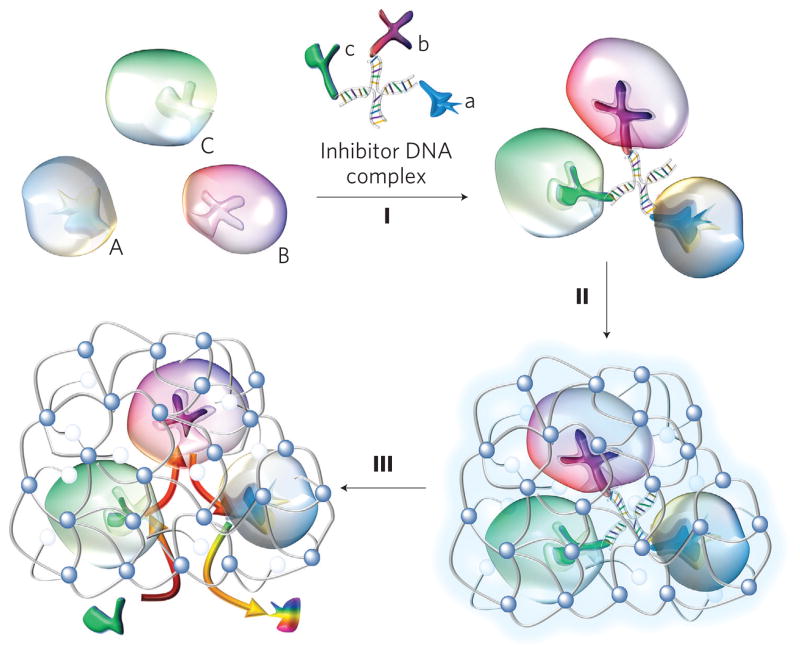
Figure 1. Synthesis of enzyme nanocomplexes.
Schematic illustration of the synthesis of a model triple-enzyme nanocomplex by DNA-directed assembly and nano-encapsulation. Spontaneous assembly of invertase (Inv, A), glucose oxidase (GOx, B) and horseradish peroxidase (HRP, C) with an inhibitor-DNA scaffold containing their respective competitive inhibitors—lactobionic acid (a), glucosamine (b) and 4-dimethylaminoantipyrine (c)—leading to the formation of a triple-enzyme architecture (I). Confinement and stabilization of the triple-enzyme architecture by in situ growth of a thin network polymer around the enzyme nanocomplex (II). Removal of the DNA scaffold leading to the formation of triple-enzyme nanocomplexes with significantly enhanced stability and close-proximity definition. Such a close-proximity architecture enables active transport of their reaction intermediates among the enzymes, leading to significantly enhanced reaction efficiency and complementary function, such as the capability to eliminate toxic intermediates (III).