Figure 1.
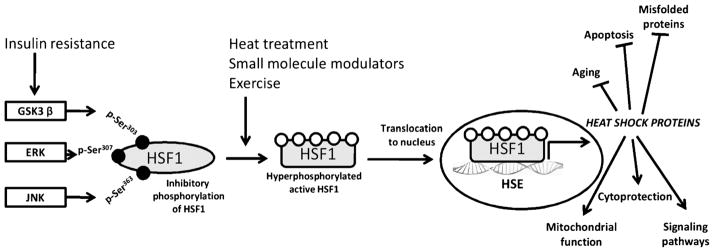
Regulation of the HSP defense mechanism. Stress (physical/chemical), change in physiological or pathophysiological states, or small-molecule activators such as bimoclomol/BGP-15 cause activation of the master regulator of the heat stress response, HSF-1. HSF-1 is a transcription factor maintained in the cytoplasm under tight negative regulation by phosphorylation. Phosphorylation by stress kinases GSK-3β, ERK, and JNK occurs on serine residues (Ser) 303, 307, and 363, respectively. The heat stress response is diminished in insulin-resistant tissue, where the activity of these kinases is increased, causing HSF-1 to remain inactive in the cytoplasm. Activation of HSF-1 results in nuclear translocation, binding to HSE, and transcription of new HSPs. HSPs function as mediators of the HSF-HSP defense system by reducing aging processes and apoptosis, enhancing signaling pathways and cytoprotection, and preventing misfolding of proteins. Stress kinases present a therapeutic target to improve the HSP defense response. HSF-1, heat shock factor 1; GSK-3β, glycogen synthase kinase 3; ERK, extracellular regulated kinase; JNK, c-Jun NH2-terminal kinase; HSE, heat shock elements; HSP, heat shock proteins.
