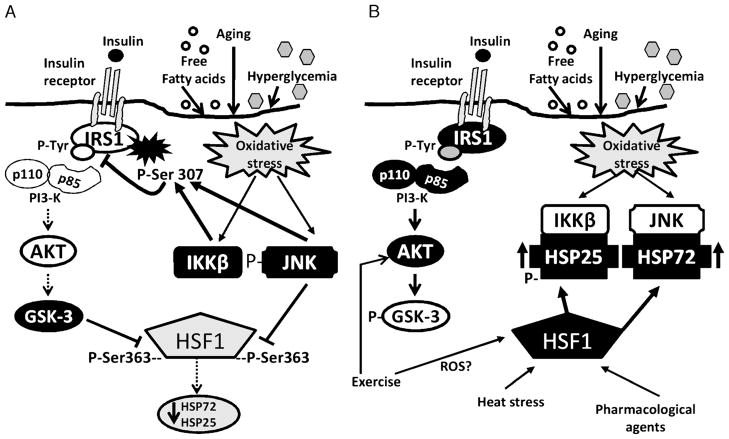
Figure 6.
A. Inhibitory stress kinase regulation of insulin signaling and heat shock protein (HSP) expression. Oxidative stress in muscle activates stress kinases c-Jun NH2-terminal kinase (JNK) and inhibitor of nuclear factor (IKKβ). These stress kinases serine phosphorylate the insulin receptor substrate-1 (IRS-1) and decrease insulin signaling by blocking IRS-1 tyrosine phosphorylation. Decreased signaling through Akt leads to increased activation of glycogen synthase kinase 3 (GSK-3), which can feedback and further increase IRS-1 serine phosphorylation. GSK-3 and JNK also can serine phosphorylate the HSP transcription factor heat shock factor 1 (HSF-1), on serine residues (Ser) 303 and 363, respectively. Chronic serine phosphorylation of HSF-1 by GSK-3 and JNK can inhibit HSF-1 and the HSP response. A decreased HSP response could result in the development and/or acceleration of a number of oxidative stress-related disease states, including insulin resistance. Open shapes represent inactive proteins, dark filled shapes represent active proteins. B. Modulating HSPs to inhibit stress kinases and improve insulin signaling. Heat stress, exercise, and pharmacological interventions increase HSPs expression in skeletal muscle. Exercise could stimulate HSP expression through a pathway independent of heat stress, whereas GSK-3–dependent activation of HSF-1 may be a common pathway of HSP activation. These pathways indicate the complex interregulatory relationship of stress kinases, insulin signaling, and HSP in skeletal muscle. Open shapes represent inactive proteins, dark filled shapes represent active proteins. ROS, reactive oxygen species.