Fig. 7.
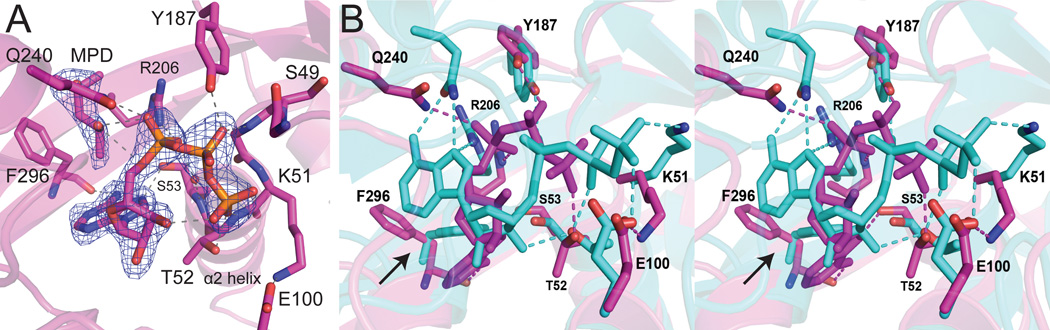
Structural basis for anion inhibition of LpxK (A) The “compact” P-loop conformation along with a chloride ion (orange sphere) and water molecule (red sphere) likely display an inactive P-loop conformer. Electron density calculated using coefficients 2Fo – Fc contoured at 2 σ is shown (mesh). To note the P-loop shift, the apo LpxK cartoon is overlaid (gray) (PDB: 4EHX) (B) Salt dependence of LpxK activity. The standard assay was performed in the presence of 0 to 0.8 M NaCl or KBr. Though having some salt (either NaCl or KBr) in the assay condition is stimulatory, excess salt becomes inhibitory. This result may represent the biochemical consequence of the “compact” P-loop conformation observed with a chloride ion obstructing the active site.