Abstract
Introduction
Deep hypothermic circulatory arrest for adult aortic arch repair is still associated with significant mortality and morbidity. Furthermore, there is still significant variation in the conduct of this complex perioperative technique. This variation in deep hypothermic circulatory arrest practice has not been adequately characterized and may offer multiple opportunities for outcome enhancement. The hypothesis of this study was that the current practice of adult deep hypothermic circulatory arrest in China has significant variations that might offer therapeutic opportunities for reduction of procedural risk.
Methods
An adult deep hypothermic circulatory arrest questionnaire was developed and then administered at a thoracic aortic session at the International Cardiothoracic and Vascular Anesthesia Congress convened in Beijing during 2010. The data was abstracted and analyzed.
Results
The majority of the 56 respondents were anesthesiologists based in China at low-volume deep hypothermic circulatory arrest centers. The typical aortic arch repair had a prolonged deep hypothermic circulatory arrest time at profound hypothermia. The target temperature for deep hypothermic circulatory arrest was frequently measured distal to the brain. The most common perfusion adjunct was antegrade cerebral perfusion, typically monitored with radial arterial pressure and cerebral venous oximetry. The preferred neuroprotective agents were steroids and propofol.
Conclusions
The identified opportunities for outcome improvement in this delineated deep hypothermic circulatory arrest model include nasal/tympanic temperature measurement and routine cerebral perfusion, preferably with unilateral antegrade cerebral perfusion monitored with radial artery pressure and cerebral oximetry. Development and dissemination of an evidence-based consensus would enhance these practice-improvement opportunities.
Keywords: adult aortic arch repair, deep hypothermic circulatory arrest, China, temperature monitoring, retrograde cerebral perfusion, antegrade cerebral perfusion, steroids, propofol, neuroprotection, cerebral oximetry, questionnaire, practice variation, radial artery pressure, cardiopulmonary bypass
INTRODUCTION
Deep hypothermic circulatory arrest (DHCA) has become a worldwide specialized technique within cardiopulmonary bypass for adult aortic arch repair [1].
This technique provides complete visualization of the aortic arch in a bloodless field with profound neuroprotection through deep hypothermia [1, 2].
Deep hypothermia exponentially decreases cerebral metabolic rate, permitting the cessation of brain perfusion for aortic arch repair. Since the cerebral metabolic rate is not zero during DHCA, there is a risk of significant neurological ischemia, especially with DHCA durations above ( 30-40 ) minutes in adults [3, 4].
In order to minimize this cerebral risk during DHCA, perfusion adjuncts such as antegrade cerebral perfusion (ACP) and retrograde cerebral perfusion (RCP) were introduced into clinical practice [5,6,7]. Furthermore, multiple pharmacologic agents has been utilized for augmented neuroprotection during DHCA, despite the lack of definitive evidence for outcome benefit [8, 9]. Given these variables in DHCA, it is therefore possible that the conduct of adult aortic arch repair can significantly vary between institutions.
Although DHCA for adult aortic arch repair was first reported in 1975 by Griep, significant variation in the conduct of DHCA has developed amongst thoracic aortic centers around the world [10,11,12]. There are still no clinical guidelines for the practice of this specialized CPB technique in adults [9]. Controversy about the conduct of DHCA for optimal neuroprotection has persisted with a lack of consensus and a paucity of randomized controlled trials [9,10,11,12,13]. The clinical studies of adult aortic arch repair with DHCA typically focus on individual institution experience. There remains a clinical gap in the definition of best DHCA practice based on the evidence and expert consensus, although the development of international registries has recently begun to address these multiple limitations [14,15,16,17].
In light of the above considerations, the purpose of this study was to evaluate the current practice of aortic arch repair in China to assess practice variations and determine opportunities for outcome improvement. The hypothesis of this study was that identified variations in the contemporary practice of DHCA for adult aortic arch repair in China might offer future opportunities for optimization of clinical outcomes.
METHODS
A 10-item questionnaire was developed by the authors to assess conduct of DHCA for adult aortic arch repair (see Appendix 1). The questionnaire, formulated both in English and Chinese, was administered at a leading thoracic aortic session at the 12th International Congress of Cardiothoracic and Vascular Anesthesia (ICCVA) held during 2010 in Beijing, China. All completed surveys were then abstracted and analyzed in an electronic database. The data was summarized in tabular and graphic formats. The design and limited administration of the questionnaire precluded complex statistical analysis.
No patient or respondent identifiers were collected: participation in this study was on an anonymous basis. The study was granted expedited approval by the institutional review board at the University of Pennsylvania. In view of the observational nature of the study, subject consent was waived as well.
RESULTS
A total of 56 respondents completed the questionnaire (Table 1).
Table 1.
Characteristics of questionnaire respondents (N=56).
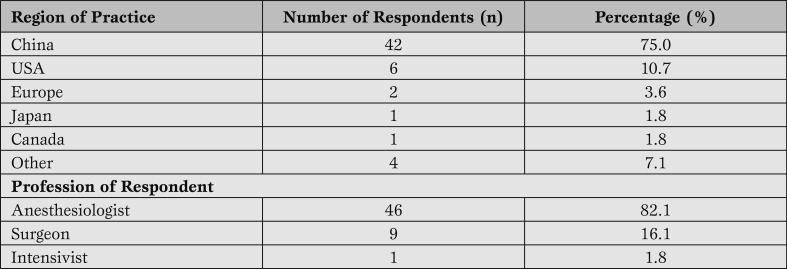
The majority of study participants were anesthesiologists who worked professionally in China. This was not a surprising observation, given that 79% of the 2010 ICCVA attendees were anesthesiologists. The majority of respondents were from low-volume surgical centers with less than one adult aortic arch repair per week. A minority of respondents were from high-volume centers with more than 100 adult aortic arch repairs per year (Figure 1).
Figure 1.
Number of adult aortic arch procedures performed per year.
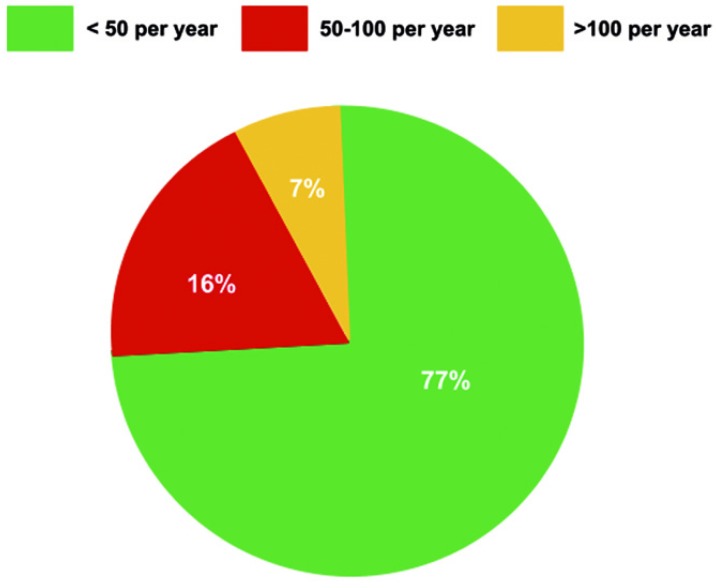
The mean DHCA time typically exceeded 30 minutes and frequently extended beyond 45 minutes (Figure 2).
Figure 2.
Typical adult aortic arch repair times.
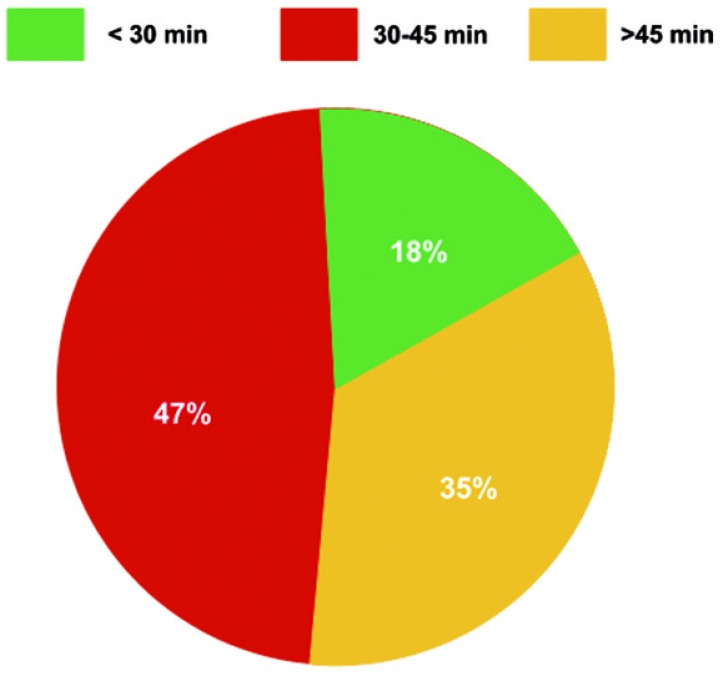
The degree of hypothermia selected for adult aortic arch repair with DHCA was typically profound with the majority of aortic centers conducting DHCA at temperatures below 20 degrees Celsius (Figure 3).
Figure 3.
Depth of hypothermia during adult aortic arch repair.
Aortic arch repair at temperatures above 20 degrees Celsius was uncommon.
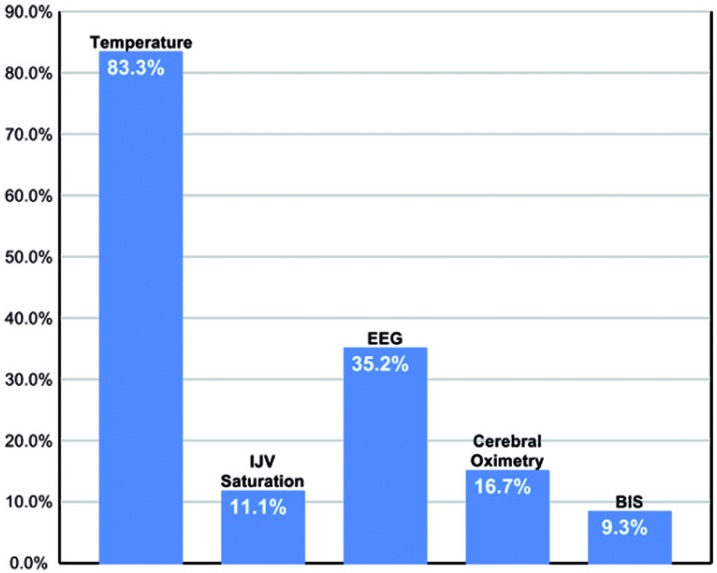
The typical clinical endpoint for cooling in DHCA was a set temperature with clinical adjuncts including electroencephalography, cerebral oximetry, internal jugular venous saturation, and bispectral index monitoring (Figure 4).
Figure 4.
Clinical endpoints for systemic cooling before Initiation of deep hypothermic circulatory arrest. IJV = Internal Jugular Vein; EEG = Electroencephalography; BIS = Bispectral Index Monitoring.
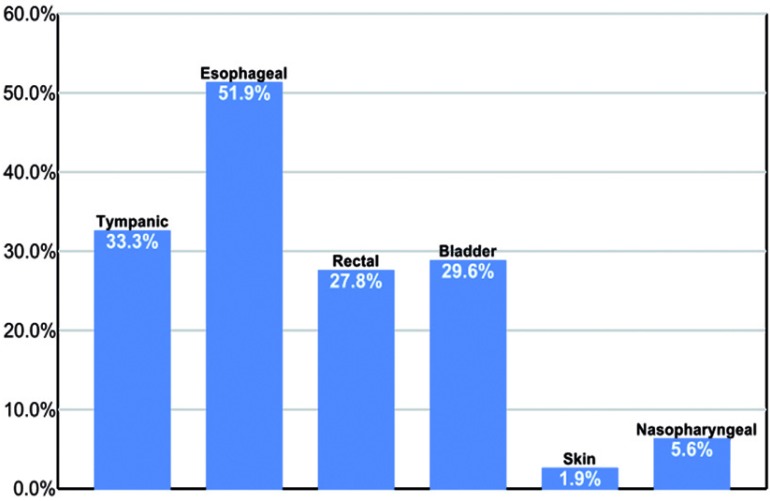
The clinical site of temperature measurement varied widely with more than 60% of centers measuring temperature distal to the brain as reflected by their exclusion of tympanic and/or nasopharyngeal sites in their DHCA temperature monitoring profiles (Figure 5).
Figure 5.
Frequency of temperature monitoring sites for quantification of brain hypothermia in adult deep hypothermic circulatory arrest.
The reasons for this divergence of practice were not apparent.
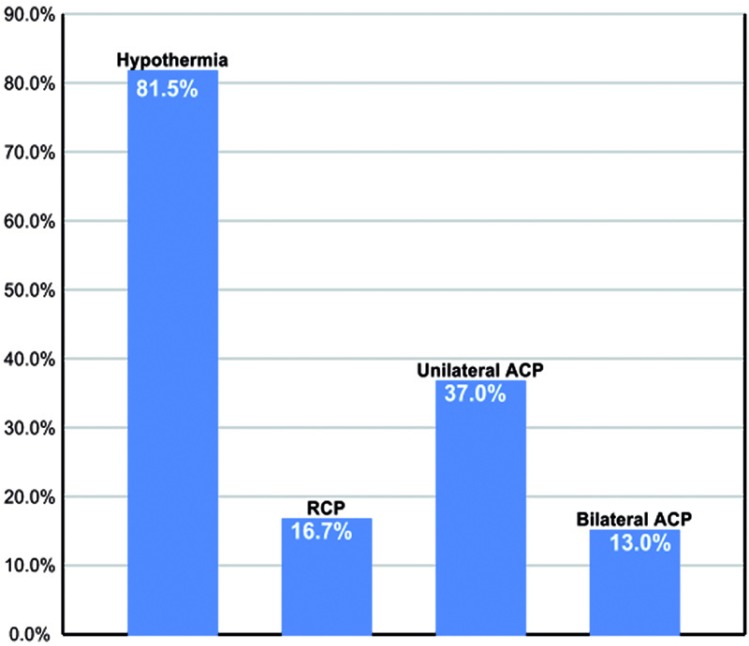
Cerebral perfusion adjuncts for neuroprotection during DHCA were collectively utilized at 2/3 of aortic centers as follows: RCP 16.7%, unilateral ACP 37.0%, and bilateral ACP 13% (Figure 6).
Figure 6.
Frequency of cerebral perfusion adjuncts during adult aortic arch repair. RCP = retrograde cerebral perfusion ; ACP = antegrade cerebral perfusion.
Given the typically extended DHCA times, these cerebral perfusion adjuncts appear to be collectively underutilized, given that they were absent in a third of centers. The reasons for this divergence in conduct of cerebral perfusion were not apparent.
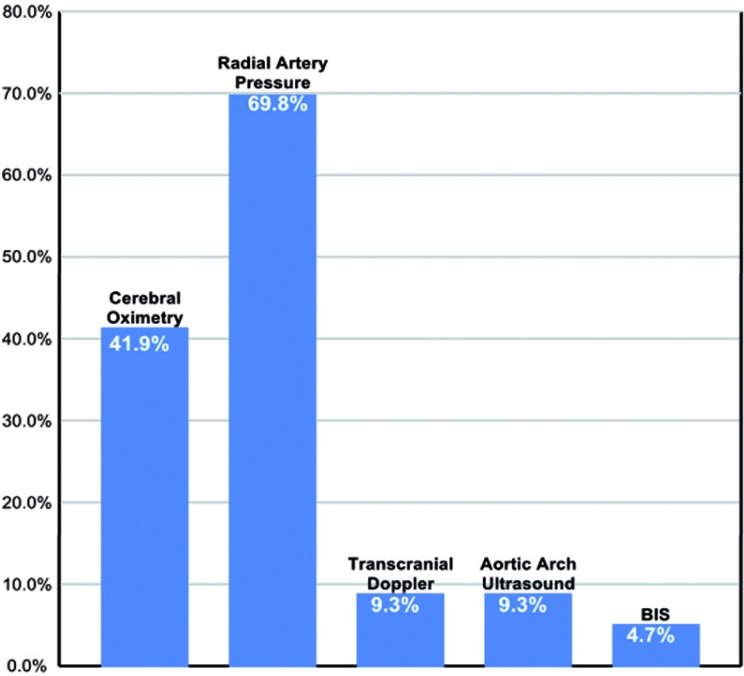
Clinical monitoring of ACP during aortic arch repair was predominantly with radial pressure monitoring and cerebral oximetry. Transcranial Doppler, ultrasound of aortic arch, and bispectral index monitoring were rarely utilized (Figure 7).
Figure 7.
Frequency of various monitors during antegrade cerebral perfusion. BIS = Bispectral Index Monitoring.
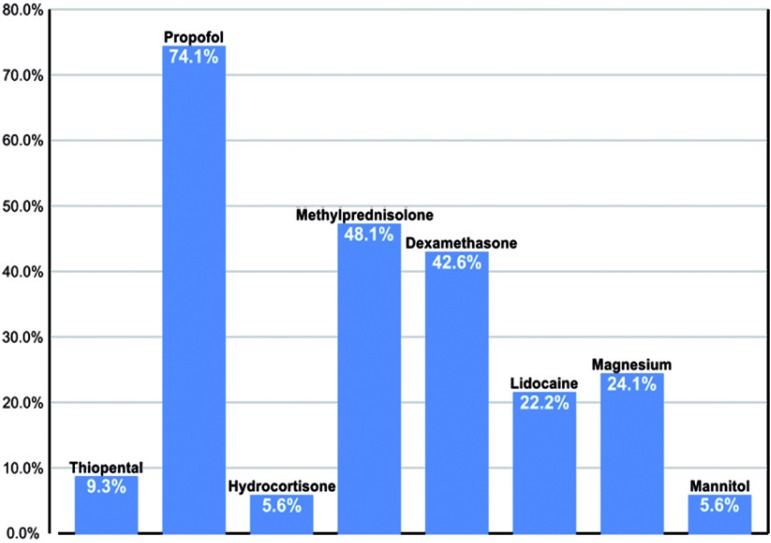
While a variety of pharmacologic agents were frequently employed as neuroprotective adjuncts, steroids collectively were a universal choice. Propofol administration was also very common (Figure 8).
Figure 8.
Frequency of various agents administered for neuroprotection during adult aortic arch repair.
DISCUSSION
This questionnaire study has sampled contemporary conduct of DHCA around China, principally in low-volume adult DHCA centers. Low-volume medical centers in China tend to perform adult aortic arch repair at profound hypothermia, defined as a temperature below 20 degrees Celsius being the common clinical endpoint for cooling. The preferred sites for temperature measurement appear, however, to be distal to the brain. Furthermore, DHCA times are commonly prolonged. There is considerable variation in the choice and conduct of adjunctive cerebral perfusion. The most common neuroprotective agents for DHCA are steroids and propofol. In this described DHCA practice model, there are opportunities for evidence-based practice improvement which will now be discussed in detail, taking the literature into account.
The site of temperature measurement that best correlates with direct brain temperature matters in adult aortic arch repair, given that hypothermia is a potent neuroprotective strategy during DHCA [18]. While rectal and bladder sites reliably reflect core body temperature, they are too remote to track cerebral temperature kinetics accurately during the cooling and rewarming phase before and after DHCA [19,20]. Although the esophagus was also a preferred site for temperature measurement, its accuracy as a surrogate for brain temperature is also impaired to its proximity to the heart and aorta, rendering it too reactive and reflective of blood and/or cardioplegia temperature [20,21,22].
Multiple studies have suggested that monitoring of the tympanic and/or nasal temperatures best approximate brain temperature [23,24,25,26,27,28,29]. A major finding from our study is that neither of these two important temperature sites are monitored in more than 60% of adult aortic arch repairs with DHCA in China. The typical selected site for clinical temperature measurement during surgery is distal to the brain, significantly raising the risk of underestimating the extent of brain hypothermia during DHCA. This management technique may result in undercooling of the brain before DHCA resulting in suboptimal neuroprotection. This practice may also result in cerebral hyperthermia after DHCA resulting in suboptimal cerebral reperfusion and risk of neurological injury [30,31,32]. Based on these considerations, it is reasonable suggest that tympanic or nasopharyngeal temperature be measured as a surrogate for brain temperature during adult cardiopulmonary bypass for DHCA and adult aortic arch repair [31, 32]. The ongoing variation in temperature management is consistent with recent data from adult general cardiopulmonary bypass [33].
Given the prolonged DHCA times identified in this study, the conduct of DHCA for neuroprotection is importantly, given that prolonged DHCA time independently predicts for mortality, stroke and major delirium after adult aortic arch repair [34, 35]. Although the optimal neuroprotective strategy remains controversial, plain DHCA beyond 40 minutes without cerebral perfusion has a stroke rate >10% in expert hands [36, 37]. These prolonged DHCA times identified in this study are associated with low-volume aortic centers and therefore low-volume aortic surgeons who would be expected to take longer to repair the aortic arch. In this setting, routine cerebral perfusion is a reasonable step to optimize neuroprotection [35]. This would be reasonably straightforward, since at least 2/3 of centers are already familiar with these techniques.
Although cerebral perfusion adjuncts were frequently reported in this study, unilateral ACP was the preferred technique. Since DHCA times are prolonged, expansion of this technique would optimize cerebral protection, since ACP appears to be the most neuroprotective in extended aortic arch repair times [1, 3, 12, 35]. Furthermore, given that it is already the predominant technique and that it is technically more straightforward than bilateral ACP, adoption of unilateral ACP as the routine cerebral perfusion during DHCA would be likely to succeed, based on the findings of this study. Unilateral ACP via the right axillary artery is a mature technique that significantly improves important outcomes in aortic arch surgery [38,39,40].
The monitoring of ACP in this study consisted predominantly of two modalities, namely radial artery pressure and cerebral oximetry. Cerebral oximetry was reported as a monitor in less than 50% of cases. Multiple studies have demonstrated that cerebral oximetry enhances the conduct of ACP by detecting cerebral malperfusion due to issues such as to an inadequate Circle of Willis and/or perfusion cannula malposition [41,42,43]. As a consequence of these outcome benefits, cerebral oximetry has already become a routine monitor for ACP at leading thoracic aortic centers [29, 30]. Based on the recent literature and the findings of our DHCA conduct study, cerebral perfusion by means of unilateral ACP with monitoring of radial arterial pressure and cerebral oximetry should be encouraged as a clinical routine for adult aortic arch repair in China.
The wide array and collective high frequency of neuroprotective agents administered for DHCA throughout China agrees with the findings of a questionnaire study from the United Kingdom [8]. In this English study, 83% of cardiac anesthesiologists administered a neuroprotective drug during DHCA. Steroids remain a popular choice in England and China. Furthermore, the English study documented significant variations in drug dose, timing of administration and physician conviction about the evidence supporting these agents [8]. The paucity of evidence complicates the development of a ‘best practice’ recommendation about these agents as a whole [8, 9]. Their endorsement would have to be based on a consensus of expert opinion.
This questionnaire study has the multiple limitations of a pilot study. The number of respondents was small. The number of study respondents does not necessarily represent the same number of institutions. A percentage of the respondents did not work primarily in China. Despite these limitations, the trends in the conduct of DHCA at low-volume centers in China were apparent.
CONCLUSION
Although this pilot study is limited and underpowered, it is an initial step in the evaluation of current DHCA practice for adult aortic arch repair in China. The survey results provide a snapshot that offers a summary of the practice of adult aortic arch repair at low-volume centers in China. Adult aortic arch repair in China is typically performed at profound hypothermia with a temperature below 20 degrees Celsius as the preferred clinical end-point. Current sites for temperature measurement are predominantly distal from the brain. The DHCA times are typically extend beyond 30 minutes. Adjunctive cerebral perfusion remains underutilized. The common neuroprotective agents for DHCA are steroids and propofol.
In this described DHCA practice model, there are opportunities for practice improvement. Temperature should be measured routinely more proximal to the brain with the preferred options including the tympanic and nasal sites.
Cerebral perfusion should be routine during DHCA, with an emphasis on unilateral antegrade cerebral perfusion due to its current clinical penetration, efficacy and technical ease. Monitoring of antegrade cerebral perfusion during DHCA should routinely include radial arterial pressure and cerebral oximetry. This study has identified opportunities for practice improvement for the conduct of adult DHCA in China. The development and dissemination of a consensus-based guideline would enhance this process significantly.
Footnotes
Source of Support Nil.
Disclosures None declared.
Cite as: Augoustides JGT, Patel P, Ghadimi K, Choi J, Yue Y, Silvay G. Current conduct of deep hypothermic circulatory arrest in China. HSR Proc Intensive Care Cardiovasc Anesth. 2013; 5 (1): 25-32.
References
- Svyatets M, Tolani K, Zhang M. et al. Perioperative management of deep hypothermic circulatory arrest. J Cardiothorac Vasc Anesth. 2010;24:644–655. doi: 10.1053/j.jvca.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Gega A, Rizzo J, Johnson M H. et al. Straight deep hypothermic arrest: experience in 394 patients supports its effectiveness as a sole means of brain preservation. Straight deep hypothermic arrest: experience in 394 patients supports its effectiveness as a sole means of brain preservation. 2007;84:759–767. doi: 10.1016/j.athoracsur.2007.04.107. [DOI] [PubMed] [Google Scholar]
- Harrington D K, Fragomeni F, Bonser R S. et al. Cerebral perfusion. Ann Thorac Surg. 2007;83:799–804. doi: 10.1016/j.athoracsur.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Augoustides J G, Floyd T F, McGarvey M L. et al. Major clinical outcomes in adults undergoing thoracic aortic surgery requiring deep hypothermic circulatory arrest: quantification of organ-based perioperative outcome and detection of opportunities for perioperative intervention. J Cardiothorac Vasc Anesth. 2005;19:446–452. doi: 10.1053/j.jvca.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Milewski R K, Pacini D, Moser G W. et al. Retrograde and antegrade cerebral perfusion: results from short elective arch reconstructive times. Ann Thorac Surg. 2010;89:1448–1457. doi: 10.1016/j.athoracsur.2010.01.056. [DOI] [PubMed] [Google Scholar]
- Apostolakis E, Akinosoglou K. The methodologies of hypothermic circulatory arrest and of antegrade and retrograde cerebral perfusion for aortic arch surgery. Ann Thorac Cardiovasc Surg. 2008;14:138–148. [PubMed] [Google Scholar]
- Bhan A, Chaudhary S H, Sharma R. et al. Modified circuit for retrograde cerebral perfusion. Asian Cardiovasc Thorac Ann. 2003;11:85–86. doi: 10.1177/021849230301100124. [DOI] [PubMed] [Google Scholar]
- Dewhurst A T, Moore S J, Liban J B. et al. Pharmacological agents as cerebral protectants during deep hypothermic circulatory arrest in adult thoracic aortic surgery: a survey of current practice. Anaesthesia. 2002;57:1016–1021. doi: 10.1046/j.1365-2044.2002.02787.x. [DOI] [PubMed] [Google Scholar]
- Shuhaiber J H. Evaluating the quality of trials of hypothermic circulatory arrest aortic surgery. Asian Cardiovasc Thorac Ann. 2007;15:449–452. doi: 10.1177/021849230701500521. [DOI] [PubMed] [Google Scholar]
- Toyama M, Matsumura Y, Tamenishi A, Okamoto H. Safety of mild hypothermic circulatory arrest with selective cerebral perfusion. Asian Cardiovasc Thorac Ann. 2009;17:500–504. doi: 10.1177/0218492309342716. [DOI] [PubMed] [Google Scholar]
- Appoo J J, Augoustides J G, Pochettino A. et al. Perioperative outcome in adults undergoing deep hypothermic circulatory arrest with retrograde perfusion in proximal aortic arch repair: evaluation of protocol-based care. J Cardiothorac Vasc Anesth. 2006;20:3–7. doi: 10.1053/j.jvca.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Augoustides J G, Andritsos M. Innovations in aortic disease: the ascending aorta and aortic arch. J Cardiothorac Vasc Anesth. 2010;24:198–207. doi: 10.1053/j.jvca.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Augoustides J G. What are the clinical questions for optimal conduct of deep hypothermic circulatory arrest for adult aortic arch repair? J Cardiothorac Vasc Anesth. 2007;21:918–919. doi: 10.1053/j.jvca.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Conzelmann L O, Hoffmann I, Blettner M. et al. Analysis of the risk factors for neurological dysfunction in patients with acute aortic dissection type A: data from the German Registry of Acute Aortic Dissection type A (GERAADA). Eur J Cardiothorac Surg. 2012;42:557–565. doi: 10.1093/ejcts/ezs025. [DOI] [PubMed] [Google Scholar]
- Easo J, Weigang E, Holzl P P. et al. Influence of operative strategy for the aortic arch in DeBakey type I aortic dissection: analysis of the German Registry for Acute Aortic Dissection Type A. J Thorac Cardiovasc Surg. 2012;144:617–623. doi: 10.1016/j.jtcvs.2011.07.066. [DOI] [PubMed] [Google Scholar]
- Di Eusanio M, Trimarchi S, Patel H J. et al. Clinical presentation, management and short-term outcome of patients with type A acute dissection complicated by mesenteric malperfusion: observations from the International Registry of Acute Aortic Dissection. J Thorac Cardiovasc Surg. J Thorac Cardiovasc Surg. 2012 doi: 10.1016/j.jtcvs.2012.01.042. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Harris K M, Strauss C E, Eagle K A. et al. Correlates of delayed recognition and treatment of acute type A aortic dissection: the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2011;124:1911–1918. doi: 10.1161/CIRCULATIONAHA.110.006320. [DOI] [PubMed] [Google Scholar]
- Elefteriades J A. What is the best method for brain protection in surgery of the aortic arch? Straight DHCA. Cardiol Clin. 2010;28:381–387. doi: 10.1016/j.ccl.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Stone J G, Young W L, Smith C R. et al. Do standard monitoring sites reflect true brain temperatures when profound hypothermia is rapidly induced and reversed? Anesthesiology. 1995;82:344–351. doi: 10.1097/00000542-199502000-00004. [DOI] [PubMed] [Google Scholar]
- Eshel G M, Safar P. Do standard monitoring sites affect true brain temperature when hyperthermia is rapidly induced and reversed? Aviat Space Environ Med. 1999;70:1193–1196. [PubMed] [Google Scholar]
- Akata T, Seloguchi H, Shirozu K, Yoshino J. Reliability of temperatures measured at standard monitoring sites as an index of brain temperature during deep hypothermic cardiopulmonary bypass conducted for thoracic aortic reconstruction. J Thorac Cardiovasc Surg. 2007;133:1559–1565. doi: 10.1016/j.jtcvs.2006.11.031. [DOI] [PubMed] [Google Scholar]
- Rango M, Arighi A, Bresolin N. Brain temperature: what do we know? Neuroreport. 2012;23:483–487. doi: 10.1097/WNR.0b013e3283534a60. [DOI] [PubMed] [Google Scholar]
- Akata T, Yamaura K, Kandabashi T. et al. Changes in body temperature during profound hypothermic cardiopulmonary bypass in adult patients undergoing aortic arch reconstruction. J Anesth. 2004;18:73–81. doi: 10.1007/s00540-003-0225-1. [DOI] [PubMed] [Google Scholar]
- Purol A, Fusciardi J, Ingrand P. et al. Afterdrop after hypothermic cardiopulmonary bypass: the value of tympanic membrane temperature monitoring. J Cardiothorac Vasc Anesth. 1996;10:336–341. doi: 10.1016/s1053-0770(96)80093-0. [DOI] [PubMed] [Google Scholar]
- Amir G, Ramamoorthy C, Riemer R K. et al. Deep brain hyperthermia while rewarming from hypothermic circulatory arrest. J Card Surg. 2009:606–610. doi: 10.1111/j.1540-8191.2009.00883.x. [DOI] [PubMed] [Google Scholar]
- Kaukuntia H, Harrington D, Bilkoo I. et al. Temperature monitoring during cardiopulmonary bypass - do we undercool or overheat the brain? Eur J Cardiothorac Surg. 2004;26:580–585. doi: 10.1016/j.ejcts.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Camboni D, Phillip A, Schebesch K M, Schmid C. Accuracy of core temperature measurement in deep hypothermic circulatory arrest. Interact Cardiothorac Thorac Surg. 2008;7:922–924. doi: 10.1510/icvts.2008.181974. [DOI] [PubMed] [Google Scholar]
- Grocott H, Newman M F, Croughwell N D. et al. Continuous jugular venous versus nasopharyngeal temperature monitoring during hypothermic cardiopulmonary bypass for cardiac surgery. J Clin Anesth. 1997;9:312–316. doi: 10.1016/s0952-8180(97)00009-3. [DOI] [PubMed] [Google Scholar]
- Kiya T, Yamakage M, Hayase T. et al. The usefulness of an earphone-type infrared tympanic thermometer for intraoperative core temperature monitoring. Anesth Analg. 2007;105:1688–1692. doi: 10.1213/01.ane.0000289639.87836.79. [DOI] [PubMed] [Google Scholar]
- Shum-Tim D, Nagashima M, Shinoka T. et al. Postischemic hyperthermia exacerbates neurologic injury after deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 1998;116:780–792. doi: 10.1016/s0022-5223(98)00449-8. [DOI] [PubMed] [Google Scholar]
- Grigore A M, Murray C F, Ramakrishna H, Djaiani G. A core review of temperature regimens and neuroprotection during cardiopulmonary bypass: does rewarming rate matter? Anesth Analg. 2009;109:1741–1751. doi: 10.1213/ANE.0b013e3181c04fea. [DOI] [PubMed] [Google Scholar]
- Murphy G S, Hessel E A 2nd, Groom R C. Optimal perfusion during cardiopulmonary bypass: an evidence-based approach. Anesth Analg. 2009;108:1394–1417. doi: 10.1213/ane.0b013e3181875e2e. [DOI] [PubMed] [Google Scholar]
- Belway D, Tee R, Nathan H J. et al. Temperature management and monitoring practices during adult cardiac surgery under cardiopulmonary bypass: results of a Canadian national survey. Perfusion. 2011;26:395–400. doi: 10.1177/0267659111409095. [DOI] [PubMed] [Google Scholar]
- Svensson L, Crawford E, Hess K. et al. Deep hypothermia with circulatory arrest: determinants of stroke and early mortality in 656 patients. J Thorac Cardiovasc Surg. 1993;106:19–28. [Discussion 28-31] [PubMed] [Google Scholar]
- Kruger T, Weigang E, Hoffmann I. et al. Cerebral protection during surgery for acute aortic dissection type A: results of the German Registry for Acute Aortic Dissection Type A (GERAADA). Circulation. 2011;124:434–443. doi: 10.1161/CIRCULATIONAHA.110.009282. [DOI] [PubMed] [Google Scholar]
- Stein L H, Elefteriades J A. Protecting the brain during aortic surgery: an enduring debate with unanswered questions. J Cardiothorac Vasc Anesth. 2010;24:316–321. doi: 10.1053/j.jvca.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Gega A, Rizzo J A, Johnson M H. et al. Straight deep hypothermic circulatory arrest in 394 patients supports its effectiveness as a sole means of brain preservation. Ann Thorac Surg. 2007;84:759–766. doi: 10.1016/j.athoracsur.2007.04.107. [DOI] [PubMed] [Google Scholar]
- Haikos M E, Kerendi F, Myung R. et al. Selective antegrade cerebral perfusion via right axillary artery cannulation reduces morbidity and mortality after proximal aortic surgery. J Thorac Cardiovasc Surg. 2009;138:1081–1089. doi: 10.1016/j.jtcvs.2009.07.045. [DOI] [PubMed] [Google Scholar]
- Leshnower B, Myung R J, Kilgo P D. et al. Moderate hypothermia and unilateral selective antegrade cerebral perfusion: a contemporary cerebral protection strategy for aortic arch surgery. Ann Thorac Surg. 2010;90:547–554. doi: 10.1016/j.athoracsur.2010.03.118. [DOI] [PubMed] [Google Scholar]
- Khaladi N, Shrestha M, Meck S. et al. Hypothermic circulatory arrest with selective antegrade cerebral perfusion in ascending aortic and aortic arch surgery: a risk factor analysis for adverse outcome in 501 patients. J Thorac Cardiovasc Surg. 2008;135:908–914. doi: 10.1016/j.jtcvs.2007.07.067. [DOI] [PubMed] [Google Scholar]
- Rubio A, Hakami L, Munch F. et al. Noninvasive control of adequate cerebral oxygenation during low-flow antegrade selective cerebral perfusion on adults and infants in the aortic arch surgery. J Card Surg. 2008;23:474–479. doi: 10.1111/j.1540-8191.2008.00644.x. [DOI] [PubMed] [Google Scholar]
- Olsson C, Thelin S. Regional cerebral saturation monitoring with near-infrared spectroscopy during selective antegrade cerebral perfusion: diagnostic performance and relationship to postoperative stroke. J Thorac Cardiovasc Surg. 2006;131:371–379. doi: 10.1016/j.jtcvs.2005.08.068. [DOI] [PubMed] [Google Scholar]
- Fischer G W, Lin H M, Krol M. et al. Noninvasive cerebral oxygenation may predict outcome in patients undergoing aortic arch surgery. J Thorac Cardiovasc Surg. 2011;141:815–821. doi: 10.1016/j.jtcvs.2010.05.017. [DOI] [PubMed] [Google Scholar]