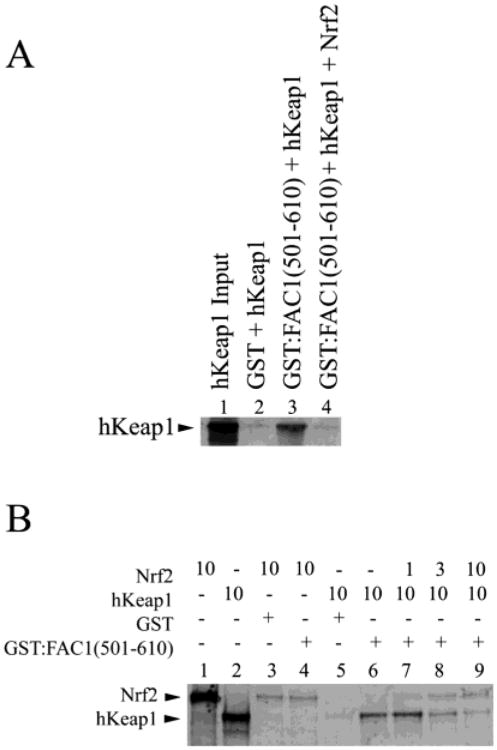
Figure 6.
FAC1 binding to hKeap1 is dramatically reduced by competition with NRF2. In (A), 35S-labeled hKeap1 (lane 1) was incubated with GST (lane 2) or GST:FAC1(501–610) in the presence (lane 4) or absence (lane 3) of in vitro translated Nrf2. Bound proteins were electrophoresed on a 10% SDS–polyacryl-amide gel, transferred to PVDF, and visualized by autoradiography. In (B), 35S-labeled, in vitro translated Nrf2 (lane 1) was incubated with GST affinity column (lane 3) or GST:FAC1(501–610) (lane 4). Shown also is the 35S-labeled, in vitro translated hKeap1 (lane 2), which is in equal amounts to Nrf2 (lane 1). A constant amount of hKeap1 (10 μL) was added to each reaction indicated. GST and hKeap1 alone (lane 5) did not interact. GST:FAC1(501–610) bound hKeap1 (lane 6) as shown in Figure 2. However, when Nrf2 was added to the GST:FAC1(501–610) binding reaction (lanes 7–9), we saw a reduction in bound hKeap1 in a dose-dependent manner. Nrf2 retention on GST:FAC1(501–610) was the same as GST control (lanes 7–9 compared to lane 3). At equimolar amounts of hKeap1 and Nrf2, >90% of the hKeap1 no longer bound FAC1.