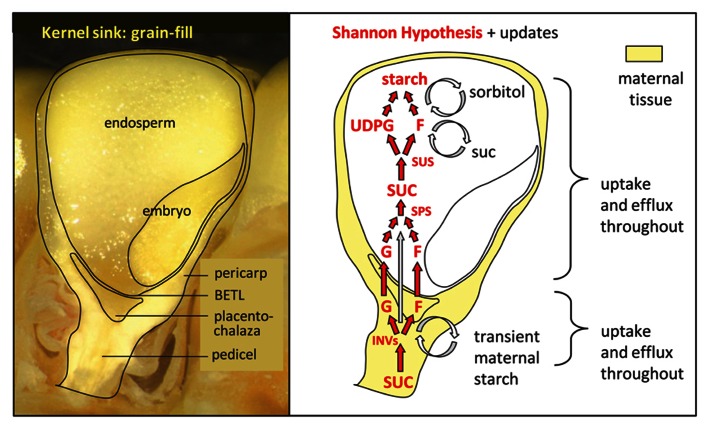
FIGURE 2.
Maize kernel sink strength during grain fill. The left panel shows a fresh, longitudinal view of a maize kernel during active starch deposition in the upper endosperm and lower pericarp (12 days after pollination). The path of assimilate entry into endosperm or embryo includes mandatory movement through the cell wall space (apoplast), because these tissues are physically isolated from the maternal structures around them by a lack of plasmodesmatal (symplastic) continuity. The right panel diagrams major contributors to sink strength relative to sites of their spatial distribution in kernels at this stage. The original Shannon hypothesis is shown in red, with updates in black. Maternal tissues are shown in yellow to emphasize the role of apoplastic transfer. Sucrose (SUC) is first cleaved by vacuolar and cell wall invertases (INV) operating in the pedicel, the placenta-chalaza, and especially by cell wall INV in the basal endosperm transfer layer (BETL). Updates to the first portion of this classical path include continual influx and efflux from maternal cells (via transporters and effluxers), and a prominent role for transient starch reserves in maternal tissues (especially the lower pericarp). Assimilates next enter the endosperm across the BETL primarily as hexoses, glucose (G) and fructose (F), but also as SUC. SUC is also resynthesized in basal portions of the endosperm by sucrose phosphate synthase (SPS), and transported to upper regions of the endosperm. Recent evidence shows extremely low oxygen levels in endosperm, which can affect several aspects of kernel sink strength. These include a limitation to metabolism of stored assimilates, advantages of sucrose resynthesis and cleavage by sucrose synthase [SUS (typically cleaving sucrose in vivo)], and cycling of sorbitol as a mechanism of redox balance under deep hypoxia. UDPG, uridine-di-phosphoglucose.