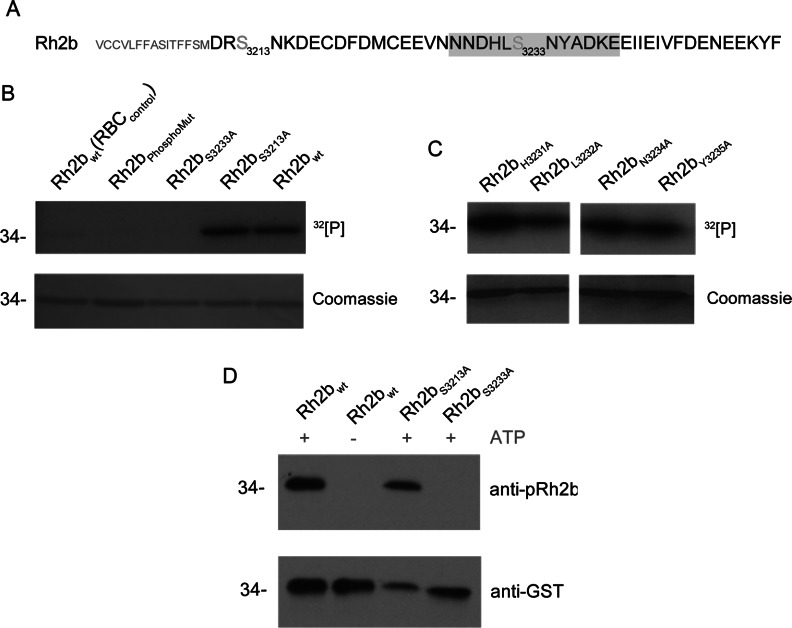
Figure 2. In vitro phosphorylation of the Rh2b mutants.
(A) Targeted amino acids in the mutational analysis are the numbered serine residues and those adjacent to Ser3233 (HL and NY). The primary sequence of the Ser3233-phosphorylated synthetic peptide used for raising an antibody (anti-pRh2b) is shaded in grey. (B) In vitro radioactive [γ-32P]ATP phosphorylation of recombinant Rh2b CPD serine mutants. Either both (Rh2bPhosphoMut) or a single serine residue were substituted with alanine. Wild-type Rh2b incubated with uninfected RBC lysate [Rh2bwt(RBCcontrol)] or with schizont lysate (Rh2bwt) are used as a negative or positive control respectively. (C) In vitro radioactive [γ-32P]ATP phosphorylation of additional point mutants targeting Ser3233 adjacent amino acids. Upper panel, autoradiography. Lower panel, Coomassie-stained SDS/PAGE. (D) Western blot analysis of recombinant wild-type and mutant Rh2b CPD that was subjected to in vitro phosphorylation in the absence or presence of ATP prior to SDS/PAGE. Western blot analysis of these proteins reveal that the anti-pRh2b antibody exclusively recognizes phosphorylated Rh2b CPD at Ser3233 (upper panel). Anti-GST antibody was used as a loading control (lower panel). The molecular mass is shown in kDa on the left-hand side of the blots.