SUMMARY
Chronic inflammation has a tight cause-and-effect relationship with DNA damage by inflicting tissue damage and increasing cancer risk. Rrm2b, a key enzyme in de novo deoxyribonucleotide synthesis, is involved in DNA damage repair, but its role in cancer development has yet to be demonstrated. Rrm2b gene loss leads to severe numerical and structural chromosome abnormalities that cause ATM activation, inducing p-Ser85 IKKγ/NEMO and IκB kinase (IKK). NF-kB consequently induced by IKK triggered sustained IL-6 expression that constitutively activates STAT3 in Rrm2b-deficient cells. High plasma IL-6 and associated hematologic disorders were observed in Rrm2b−/− mice. 30–40% of aged Rrm2b heterozygous knockout mice developed plasma cell neoplasms and suffered from progressive splenomegaly and ascites. The genetic ablation of IL-6 suppressed STAT3 induction and delayed disease onset in Rrm2b−/− mice, extending their life span. Thus, Rrm2b serves a crucial role in maintaining chromosomal stability and preventing chronic inflammation-associated tumorigenesis.
Keywords: Chronic inflammation, IL-6, DNA damage, Plasmacytic neoplasms, and Rrm2b
INTRODUCTION
Chronic inflammatory responses disrupt tissue homeostasis and promote cancer development and progression (Grivennikov et al., 2010). It was postulated that activated inflammatory cells serve as a source of reactive oxygen species (ROS) capable of inducing DNA damage and genomic instability. Conversely, DNA damage might also lead to inflammation and promote tumorigenesis (Rodier et al., 2009). Current cancer treatment by radiation therapy or DNA damaging chemotherapeutic drugs may result in inflammation that can stimulate tumor growth. Uncovering the molecules that trigger chronic inflammation associated with sustained DDR signaling could lead to a deeper understanding of cancer development and effective strategies in cancer prevention.
DNA damage triggers the DDR in cells through a signal amplification cascade (Rodier et al., 2009). The DDR in reversible DNA damage transiently arrests proliferating cells as DNA is repaired. However, cells with irreparable DNA damage, often caused by strong genotoxic stresses, present with persistent DNA damage foci (PDDF), which are detectable by the presence of 53BP1 and H2AX (Rodier et al., 2009). These cells express a wide range of chemokines and cytokines (such as IL-6, IL-8, MMP3, and CXCL1), known as the senescence-associated secretary phenotype (SASP), are mediated by ATM but not by senescence regulators. IL-6 and IL-8 corresponding to ATM expression were found in premalignant breast lesions (Rodier et al., 2009). There is increasing evidence demonstrating that DNA damage can lead to inflammation and thereby promote tumorigenesis. The murine model of hepatocellular carcinoma was induced by the carcinogen diethylnitrosamine (DEN), in which DNA damage contributes to necrotic cell death, resulting in an inflammatory reaction that promotes tumor development (Maeda et al., 2005). Defective DNA repair caused by a deficiency of the Fen1 exonuclease also results in a tumor promoting inflammatory response that is driven by damaged DNA (Zheng et al., 2007).
Ribonucleotide reductase (RR) plays an essential role in supplying deoxyribonucleoside triphosphate (dNTPs) for DNA synthesis and repair. The RR holoenzyme consists of a heterodimeric tetramer of nonidentical homodimers of large Rrm1 (R1) and small Rrm2 (R2) or Rrm2b (p53R2) subunits (Nordlund and Reichard, 2006). dNTP is provided by the R1–R2 complex in S phase. In the G0/G1 phase following DNA-damage stress, dNTPs for nuclear DNA synthesis and repair are supplied by Rrm2b (Tanaka et al., 2000). The R1-Rrm2b complex also contributes dNTPs for basal DNA repair and mitochondrial DNA synthesis in quiescent cells without external DNA damage stress (Bourdon et al., 2007). Despite evidence supporting Rrm2b’s involvement in DNA damage repair, its role in cancer development in vivo is not yet defined.
Rrm2b can also be regulated at the post-translational level by ATM during the acute phase of the genotoxic stress response and is associated with the MRE11 complex (Chang et al., 2008). Our earlier findings linking Rrm2b to ATM strongly suggest a potential role of Rrm2b in DDR and disease development. In this study, we investigated this possibility using Rrm2b knockout mice. Rrm2b homozygous knockout (Rrm2b−/−) mice did not live long enough for cancer to be observed. In contrast, Rrm2b heterozygous knockout (Rrm2b+/−) mice are developmentally normal and fertile. Some Rrm2b+/− mice developed chronic inflammation and plasma cell neoplasia after nine months of age and showed signs of morbidity. This study is the first to show that Rrm2b-deficient mice provide an important murine model for understanding chronic inflammatory states as well as neoplastic diseases in the context of persistent DDR signaling and chronic inflammatory cytokine secretion.
RESULTS
Constitutive NF-KB and STAT3 Mediate Sustained IL-6 Expression in Rrm2b-Deficient Cells
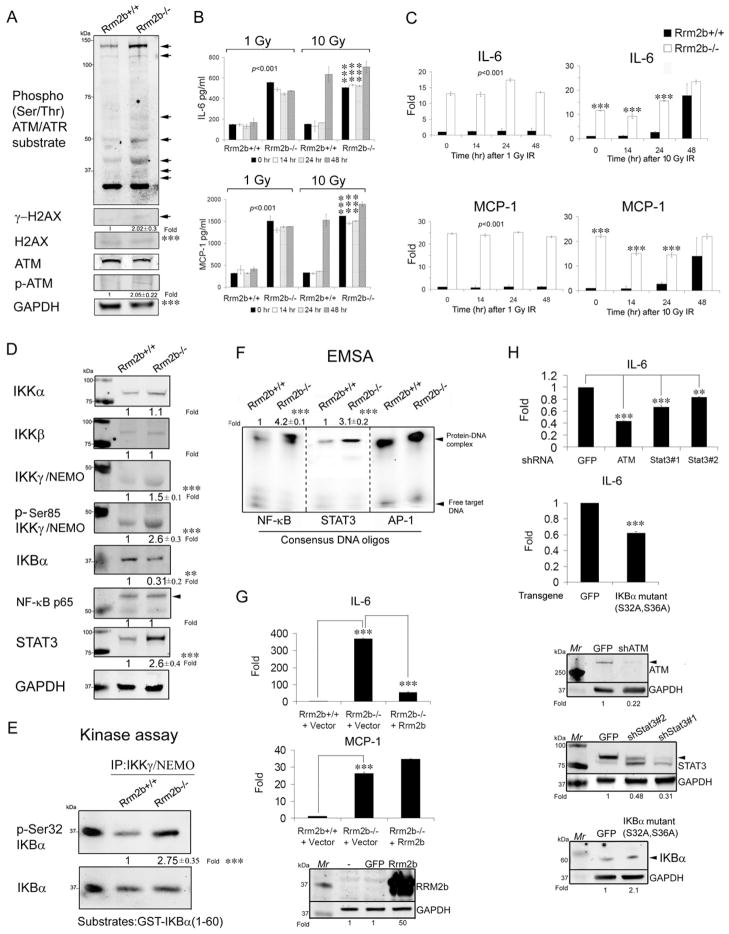
To address whether RRM2b participates in ATM signaling in response to genotoxic stress, we first compared the activities of ATM and its downstream substrates between Rrm2b+/+ and Rrm2b−/− primary MEFs by immunoblot (IB) analysis. Activated ATM and γH2AX were more apparent in Rrm2b−/− than Rrm2b+/+ wild-type (WT) MEFs (Figure 1A). Additionally, a number of ATM/ATR substrates were more highly activated in Rrm2b−/− MEFs than in Rrm2b+/+ MEFs, as determined by an antibody directed against phosphorylated Ser or Thr residues in the ATM/ATR substrate motif. ATM and NBS1 protein levels were similar between Rrm2b+/+ and Rrm2b−/− MEFs (Figure 1A). Deletion of Rrm2b also did not change the protein levels of other RR subunits, RRM1 and RRM2 (Figure S1A). The ATM signaling cascade can regulate a range of chemokines and cytokines (Rodier et al., 2009). Thus we used multiplex cytokine analysis to further analyze the cytokine/chemokine profile in culture medium from Rrm2b+/+ and Rrm2b−/− MEFs at passage-2 before and after IR exposure. No significant difference in cytokine/chemokine levels were observed between the two cell types except for interleukin-6 (IL-6) and monocyte chemoattractant protein-1 (MCP-1) (Figures 1B). Compared to Rrm2b+/+ MEFs, IL-6 and MCP-1 proteins were already 3-fold higher in Rrm2b−/− MEF media prior to IR exposure (Figure 1B). IL-6 and MCP-1 productions in WT cells can be induced by a high (10 Gy) but not by a low (1 Gy) dose of IR (Figure 1B). However, production of endogenous IL-6 and MCP-1 by Rrm2b−/− MEFs was not further induced by 10 Gy IR, indicating that Rrm2b−/− MEFs already constitutively secreted both cytokines at maximal levels. Constitutive IL-6 and MCP-1 secretion also corresponded to high endogenous amounts of IL-6 and MCP-1 RNA transcripts in Rrm2b−/− MEFs (Figure 1C). IL-6 and MCP-1 genes can be regulated by NF-κB, which has been shown to be a predominant transcription factor in mediating downstream target genes of ATM (Wu et al., 2006). We next evaluated if constitutive ATM activity in Rrm2b−/− MEFs also altered NF-κB activity. NF-κB activity is modulated by the inhibitor IKBα, which is in turn negatively regulated by the IKK complex comprised of two catalytic subunits, IKKα and IKKβ, and the scaffolding protein IKKγ/NEMO (Grivennikov et al., 2010). The levels of IKKα and IKKβ as well as total NF-κB proteins were similar between Rrm2b+/+ and Rrm2b−/− MEFs, whereas IKKγ proteins were slightly elevated in Rrm2b−/− MEFs (Figure 1D). The IKKγ/NEMO proteins were also highly phosphorylated at serine 85, which is known to be dependent on ATM in response to genotoxic stress (Wu et al., 2006). Phosphorylated IKKγ/NEMO in Rrm2b−/− MEFs caused a 2-fold higher IKK kinase activity when compared to Rrm2b+/+ MEFs (Figure 1E). Consequently, IKBα, a repressor of NF-κB activity, accumulated much less in Rrm2b−/− MEFs compared to WT (Figure 1D). Constitutive DNA binding activity of both NF-κB and STAT3 were 2–3 fold higher in Rrm2b−/− MEFs than Rrm2b+/+ MEFs (Figure 1F). mRNAs of a number of IL-6 downstream target genes, such as STAT3, c-MYC, and JAGGED1 were correspondingly elevated in Rrm2b−/− MEFs before and after exposure to IR (Figures S1B). STAT3 protein levels also accumulated more in Rrm2b−/− MEFs than Rrm2b+/+ MEFs (Figure 1D). Reconstitution of Rrm2b−/− fibroblasts with a lentivirus encoding Rrm2b gene was able to suppress the expression of IL-6, but not MCP-1 (Figure 1G). These data suggest that induction of IL-6 may represent a direct consequence of Rrm2b deficiency or a positive regulatory response to Rrm2b deficiency. ATM knockdown reduced IL-6 induction by more than 50% (Figure 1H). 50 to 70% depleted STAT3 was able to reduce IL-6 induction by 20 to 30% significantly. A significant 30% reduction of IL-6 can also be achieved by inactivation of NF-κB activity in Rrm2b−/− MEFs with forced expression of adenoviral IkBα super-repressors (IkBα S32A/S36A) (Figure 1H). We also investigated if constitutive IL-6 induction triggered by Rrm2b deficiency can be attributed to senescence. There were actually less senescent Rrm2b−/− MEFs compared to Rrm2b+/+ MEFs at both low (passage-2) and high (passage-11) passages (Figure S1C). IL-6 and MCP-1 transcription levels were even higher in Rrm2b−/− than in Rrm2b+/+ MEFs at passage-2, whereas p16 and p53 mRNAs were similar between both cell types (Figure S1D). At passage-11, both IL-6 and MCP-1 RNAs were significantly elevated in Rrm2b−/− MEFs than Rrm2b+/+ MEFs (Figure S1D). IL-6 and MCP-1 RNAs were not yet induced in high-passage Rrm2b+/+ MEFs despite increased senescence. Rrm2b−/− MEFs also showed increased cyclin D1 proteins (Figure S1F) and demonstrated rapid cellular growth (Figure S1E). Knockdown of senescence regulator p16 (Figure S1G) or p53 (Figure S1H) resulted in slightly increased IL-6 in Rrm2b−/− MEFs. Taken together, in the context of Rrm2b deficiency, IL-6 induction through NF-κB and STAT3 depended on ATM but not senescence regulators (p16 and p53).
Figure 1. Sustained IL-6 expression mediated by constitutive NF-κB and STAT3 in Rrm2b-deficient Cells.
(A) Immunoblotting (IB) analysis of ATM-related signaling proteins in MEFs derived from Rrm2b+/+ and Rrm2b−/− E14.5 embryos. Phosphorylated H2Ax at Ser139, γ-H2AX; Phosphorylated ATM at Ser1981, p-ATM. (B) IL-6 and MCP-1 cytokines from Rrm2b+/+ and Rrm2b−/− MEF media before and after 1 or 10 gray (Gy) IR at the indicated time-points were analyzed by Luminex multiplex cytokine assays. (C) RT-qPCR analysis of IL-6 and MCP-1 mRNAs of MEFs before and after 1 or 10 Gy IR. (D) IB analysis of NF-KB and STAT3 signaling proteins in MEFs from each genotype. (E) IKK kinase activity in MEFs of each genotype analyzed by the IKKγ–immunoprecipitated complex on GST-IKBα(1-60) recombinant proteins followed by IB with p-Ser32 IKBα and IKBα antibodies. (F) NF-kB and STAT3 DNA binding activity in MEFs from each genotype analyzed by a non-radioactive electrophoretic mobility shift assay (EMSA) after quantification and normalization with AP-1 binding activity, which served as a control for extract quality and loading. (G) RT-qPCR analysis of IL-6 and MCP-1 mRNAs in Rrm2b−/− fibroblasts after infection with lentiviral Rrm2b transgenes. Transgene proteins were examined by IB in the bottom panel. (H) RT-qPCR analysis of IL-6 mRNA in Rrm2b−/− MEFs after infection with lentiviruses expressing shRNAs against the indicated target genes or GFP (control). Knocked-down proteins were analyzed by IB and shown in the bottom panels. The fold-chang (Fold) in IB, kinase activity, and EMSA assays is a relative value to WT control after subtraction from the loading control, which given was given an arbitrary value of 1.0. Data represented as mean ± standard deviation (SD) were average from three independent MEFs derived from different embryos for each genotype. **,p< 0.01; ***,p< 0.001. See also Figure S1.
Loss of Rrm2b Gene Causes Chromosomal Instability
To directly identify chromosomal abnormality of any forms or frequencies, we analyzed MEFs by spectral karyotype (SKY) analysis (Figure 2). SKY was performed on MEFs at passage one to minimize the effects of extended cell culture passaging. The data indicated that nearly all the examined chromosomes from Rrm2b+/+ MEFs had only one break (Figures 2A and 2B). In addition to chromatid breakages, severe forms of chromosomal abnormalities, including deletion, loss, and translocation were identified in Rrm2b haploinsufficient (Rrm2b+/−) MEFs. Deletion of both Rrm2b alleles created additional chromosomal abnormalities, including gains, duplications, and isochromosomes (Figures 2A and 2B). The total spontaneous aberrations from each chromosome in Rrm2b−/− MEFs were almost 100-fold and 5-fold higher compared with Rrm2b+/+ and Rrm2b+/− MEFs, respectively (Figure 2A). After exposure to 1 Gy IR, additional forms of chromosomal abnormalities (derivative and dicentric chromosomes) were observed in Rrm2b−/− MEFs, whereas chromatid breakages were the only type of abnormality found in Rrm2b+/+ MEFs (Figure 2C). Excluding chromatid breakages, there was almost a 300-fold increase in IR-induced chromosomal aberration frequencies in Rrm2b−/− MEFs compared to Rrm2b+/+ MEFs (Figure 2A). These results demonstrate that Rrm2b is involved in preserving the integrity of chromosomal number and structure, thereby helping to maintain genetic stability. The same batch of MEFs which were analyzed for genetic abnormality in Figure 2 were also analyzed for DDR signaling and IL-6 expression at a single cell level by immunocytochemistry (ICC) (Figure S2). Compared to Rrm2b+/+ MEFs, Rrm2b−/− MEFs showed robust IL-6, p-ATM, and γH2AX signals (compare Figures S2A to S2B, S2D to S2E and S2G to S2H). Distinct nuclear p53 binding protein-1 (53BP1) foci formation accumulated robustly in Rrm2b−/− MEFs (compare Figure S2J to S2K). IL-6, p-ATM, γH2AX and 53BP-1 signals in Rrm2b+/+ MEFs were low prior to IR but higher following exposure to 4 Gy IR (Figures S2C, S2F, S2I and S 2L). Nuclear Rrm2b expression in Rrm2b+/+ MEFs (Figure S2M) appeared to be specific as it was completely absent in Rrm2b−/− MEFs (Figure 2N).
Figure 2. Increased Numerical and Structural Chromosome Abnormalities in Rrm2b Deficient Cells.
(A) The values in the Table represent the number of each different type of aberrant chromosomes in the examined total chromosomes from three independent cell lines before and 24 h after 1Gy IR for each genotype analyzed by FISH karyotype analysis. The fold-chang of chromosome aberration frequencies excluding Chtb among the genotypes was shown in the histograms. Chromatid break, Chtb; Deletion, del; Extra copies of chromosomes, gain; Missing copies of chromosomes, loss; Derivative chromosome, der; Duplicated chromosome, dup; Chromosomes having two centromeres, dicentric; Fusion of chromosomes, isochromosome. A representative pseudocolor spectral karyotype image of one MEF from each genotype before (B) and 24 h after 1 Gy IR (C). Gain, +; Loss, −; Translocation, t; Dicentric, dic. Data represented as mean ± SD. ***,p< 0.001. See also Figure S2.
Mice Deficient in Rrm2b Exhibit Elevated IL-6 and Hematologic Disorders
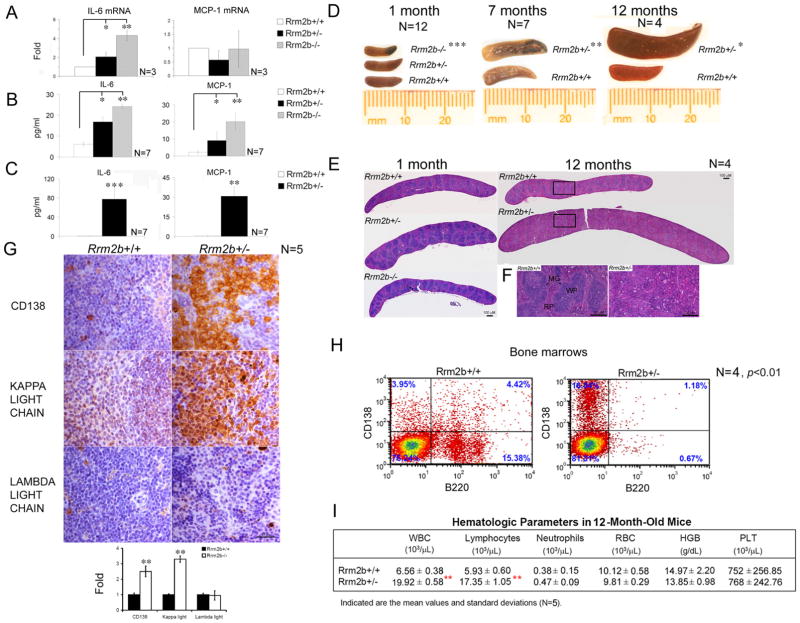
Next, we assessed whether losing the Rrm2b gene can cause a change of IL-6 and MCP-1 expression in mice tissue. Loss of the Rrm2b gene resulted in a dose-dependent effect on IL-6 gene induction in bone marrows (Figure 3A). IL-6 RNA levels in Rrm2b−/− bone marrows were four-fold higher than that of Rrm2b+/+ bone marrows, whereas IL-6 levels in Rrm2b+/− bone marrows were intermediate to those of the two genotypes (Figure 3A). Similar outcomes were observed in 1-month-old mice blood where the loss of one or both Rrm2b genes corresponded to increases in serum IL-6 and MCP-1 protein levels in a dose-dependent manner (Figure 3B). Accumulation of MCP-1 and, especially, IL-6 continued in 9- to 12-month-old Rrm2b+/− mice but not in age-matched Rrm2b+/+ littermates (Figure 3C). Unfortunately, Rrm2b−/− mice did not survive longer than seven weeks after birth. Beginning at seven months, some Rrm2b+/− mice developed severely hemorrhagic splenomegalies (Figure 3D). Therefore, we monitored various organ weights and the total body weight from the 7- to 9-month-old Rrm2b+/+ and Rrm2b+/− mice and found that found that spleens from Rrm2b+/− mice were substantially and significantly larger than those from Rrm2b+/+ mice, whereas other organs were comparable in size and weight (Figure S3A). The liver was slightly enlarged in Rrm2b+/− mice as compared with the normal counterparts, but the difference between two genotypes was insignificant (Figure S3A). Histologic examination of Rrm2b+/− spleen sections at different ages also showed signs of progressive pathology. The white pulps of Rrm2b+/− and Rrm2b−/− spleens were larger and more in number than those of Rrm2b+/+ spleens at 1-month of age (Figure 3E). At 12 months, the architecture of Rrm2b+/− spleen appeared remarkably disorganized as marginal zones disappeared and the red and white pulps became indistinguishable (Figures 3F). Clusters of CD138+ (a plasma cell marker) plasmacytoid infiltrate were also observed in Rrm2b+/− spleens (Figure 3G). These compactly arranged plasmocytoid cells appeared to be monoclonal, which selectively expressed the immunoglobulin (Ig) KAPPA, but not LAMBDA, light chain (Figure 3G). Furthermore, plasma cell numbers were more than 5-fold higher in Rrm2b+/− than in Rrm2b+/+ bone marrows (Figure 3H). In contrast, less than 1% of Rrm2b+/− expressed B220, a marker of early pro-B cells, whereas approximately 15% of the Rrm2b+/+ bone marrow population were B220 positive (Figure 3H). These results indicated that most of the populations of B cells in aged Rrm2b+/− mice were plasma cells. An elevated plasma cell count should lead to an abnormal amount of antibody production. As expected, abnormal Ig levels were found in mice deficient in the Rrm2b gene (Figure S3). IgA and IgE levels were similar in young and old mice regardless of genotype (Figures S3B and S3C). However, in younger mice, IgM levels increased and IgG levels decreased significantly with the loss of Rrm2b gene in a dose-dependent manner (Figures S3D and S3E). Serum IgG levels were nearly undetectable in Rrm2b−/− mice (Figure S3E). With age, Rrm2b+/− mice developed significantly higher serum IgM levels than age-matched Rrm2b+/+ mice (Figure S3D). Conversely, IgG levels were significantly lower in aged Rrm2b+/− mice compared to Rrm2b+/+ mice (Figure S3E). Defective B cell development and abnormal serum Ig levels in Rrm2b-deficient mice prompted us to analyze the complete blood count (CBC) profiles of these mice. Significantly higher levels of WBC, lymphocyte, and neutrophil were found in 1-month-old Rrm2b−/− mice compared to Rrm2b+/+ littermates (Figure S3F). Rrm2b+/− mice had cell counts intermediate to that of Rrm2b−/− and Rrm2b+/+ littermates, indicating an Rrm2b gene dose-dependent effect in the development of blood cell lineages. Aged and sick Rrm2b+/− mice developed even more significantly elevated levels of WBC and lymphocyte counts than their WT littermates (Figure 3I). Some Rrm2b+/− mice also presented with hair loss, skin infections, and enlarged lymphoid nodes (Figures S3H, S3J and S3L). Some of sick Rrm2b+/− mice even had a ruptured spleen and liver and developed ascites close to 12 months (Figure S3M). Abnormally high monoclonal plasma cells, Ig levels and WBC counts are hallmarks of patients suffering from plasma cell neoplasms, including multiple myeloma and plasmacytoma (Naugler and Karin, 2008).
Figure 3. Elevated IL-6 and MCP-1 and the Plasma Cell Neoplasia Phenotype in Aged Rrm2b+/− mice.
IL-6 and MCP-1 (A) mRNAs in 1-month-old bone marrows from each genotype analyzed by qPCR. IL-6 and MCP-1 protein levels from blood serum of 1-month-old (B) and from 9- to 12-month-old mice (C). (D) Spleens from each genotype at the indicated ages. The data shown were obtained from a litter-matched group of mice. (E) Tiling images of representative H&E stained sections of spleen from each genotype at 1 and 12 months. Higher magnification of the boxed area of 12-month-old spleens is shown in (F) Marginal Zone, MG; White pulp, WP; Red pulp, RP. (G) A representative section of IHC staining for CD138, KAPPA light chain and LAMBDA light chain antibodies (Brown color) from 12-month-old Rrm2b+/+ and Rrm2b+/− spleens. (H) Fluorescence-activated cell sorting (FACS) of cells derived from Rrm2b+/+ and Rrm2b+/− primary bone marrows for plasma cells using CD138 and B220 antibodies. (I) CBC of peripheral blood from 12-month-old mice. White blood cell, WBC; Red blood cell, RBC; Hemoglobin, HBG; Platelet, PLT. Data represented as mean ± SD. *,p< 0.05; **,p< 0.01; ***,p< 0.001. See also Figure S3.
The Effect of Crossing Rrm2b−/− Mice with IL-6-deleted Mice
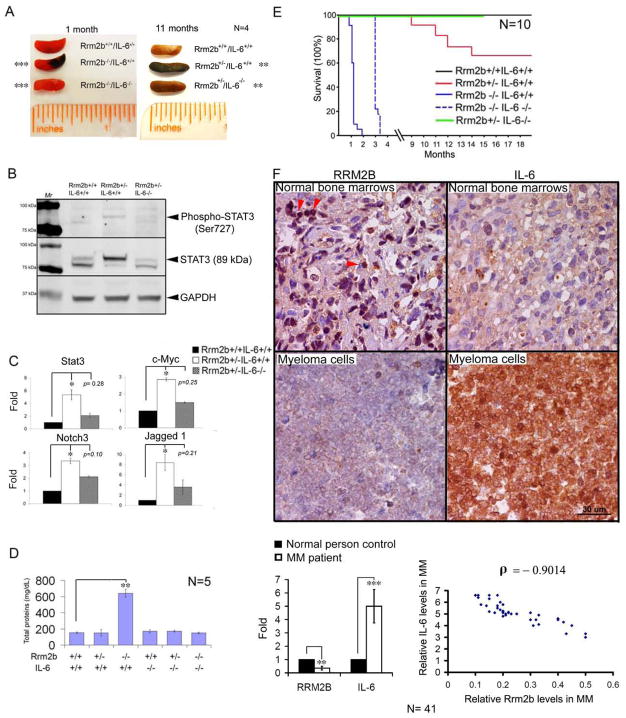
The observation that IL-6 production was increased in Rrm2b-deficient cells and mice led us to investigate the contribution of this cytokine to disease in Rrm2b−/− mice. Rrm2b+/− and IL-6+/− heterozygote mice were crossed to generate Rrm2b−/−IL-6−/− double mutants. Hemorrhaging was not apparent in 1-month-old Rrm2b−/−IL-6−/− and 11-month-old Rrm2b+/−IL-6−/− spleens (Figure 4A). Abnormal IgM (Figure 4SA) and IgG (Figure 4SB) levels in Rrm2b+/− and Rrm2b−/− mice were restored to a range similar to Rrm2b+/+ littermates after IL-6 deletion. The CBC profiles from Rrm2b−/−IL-6−/− and Rrm2b+/−IL-6−/− mice were also similar to their WT littermates (Figures S4C and S4D). Elevated STAT3 (89 kDa) proteins and phoshorylation (Ser727) levels (Figure 4B) as well as mRNAs of IL-6 downstream genes (STAT3, c-Myc, Notch, and Jagged 1) (Figure 4C) in old Rrm2b+/−IL-6−/− bone marrows were suppressed following IL-6 deletion. The removal of IL-6 dramatically reduced urine protein secretion and glomerulopathy (Figures 4D and S4E). Despite extensive genomic abnormalities in Rrm2b−/− MEFs, elimination of the IL-6 gene was still able to extend the life of Rrm2b−/− mice by 8 to 10 weeks (Figure 4E). The mortality of Rrm2b+/−IL-6−/− mice was undetectable at 9 to 14 months compared to 30–40% in Rrm2b+/−IL-6+/+ mice of the same age (Figure 4E). Most importantly, bone marrow myeloma cells of multiple myeloma patients (N=41) showed a significantly 3-fold decrease in Rrm2b and 5-fold increase in IL-6 expression compared to those of normal human bone marrows. Rrm2b levels inversely correlated with IL-6 expression in MM patients (the ρ values of Pearson’s correlation coefficient = − 0.9014).
Figure 4. IL-6 Deficiency Delayed Disease Onset and Mortality of Rrm2b−/− mice.
(A) Absence of splenic hemorrhage in the representative 1-month-old Rrm2b−/−IL-6−/− spleen and 11-month-old Rrm2b+/−IL-6−/− spleen compared to their age-matched littermates. (B) Analysis of STAT3 activity and protein expression in 12-month-oldbone marrows with indicated genotypes by IB analysis using antibodies against phospho-STAT3 (Ser727) and STAT3. (C) mRNA levels of the indicated genes in 12-month-old bone marrows with indicated genotypes. (D) Deletion of IL-6 blocked urinary protein secretion in Rrm2b−/− mice at 5 weeks of age. (E) Kaplan-Meier survival curve of mice with indicated genotypes. (F) A representative section of IHC staining for Rrm2b and IL-6 antibodies (Brown color) from normal human bone marrows and myeloma cells of a MM patient (N=41). Nuclear Rrm2b indicated by arrow heads. The quantification of Rrm2b and IL-6 signals was shown in the histogram. Correlation between relative IL-6 and Rrm2b levels in MM patients was plotted and analyzed by Pearson correlation coefficient analyses. ρ denotes Pearson’s correlation. Data represented as mean ± SD. *,p< 0.05; **,p< 0.01; ***,p< 0.001. See also Figure S4.
DISCUSSION
DNA damage caused by depleted dNTP pools can trigger ATM-Chk2-like S-phase checkpoint pathway (Kumar et al., 2011). Elevated ATM activity induced by attenuated dNTP pools in Rrm2b-deficient MEFs might serve as a genome surveillance mechanism. In addition to orchestrating cell-cycle checkpoints, ATM stimulates the secretion of proinflammatory cytokines induced by irreparable DNA damage (Rodier et al., 2009). Chromosomal abnormalities in Rrm2b-deficient MEFs (Figure 2) could have led to irreparable DNA damage and constitutive DDR signaling, resulting in the autonomous secretion of cytokines, including IL-6 and MCP-1 (Figure 1). Our data confirms previous findings that persistent DDR-induced IL-6 mRNA in Rrm2b−/− MEFs can be suppressed greater than 50% by shRNA against ATM (Figure 1). Rrm2b was previously shown to be associated with the ATM and the MRN complex (Chang et al., 2008). In addition to supplying dNTPs, Rrm2b might interact with the ATM and the MRN complex to maintain chromatin stability. Therefore, constitutive ATM activation by Rrm2b deficiency may be potentially attributed to a mechanism independent of dNTP depletion.
NF-κB and STAT3 are central signalling hubs in regulating inflammation-mediated tumour promotion and metastasis (Grivennikov et al., 2010). Cooperative signaling through the NF-κB and STAT3 pathways has been found in numerous cancers. Although through distinct mechanisms, activated NF-κB and STAT3 can either synergistically or individually control the expression of anti-apoptotic, pro-proliferative, and proinflammatory cytokine genes to promote tumorigenesis. IL-6 has been implicated as a potential mediator between NF-κB and STAT3 ( Grivennikov and Karin, 2008). Here we demonstrate that aberrant activation of NF-κB induced by ATM-IKK led to the stimulation of STAT3 and consequent IL-6 release, providing novel evidence of a cross-talk between NF-κB and STAT3 signaling in the context of genome instability triggered by Rrm2b deficiency. The interplay between NF-κB and STAT3 might make it difficult to completely blunt IL-6 induction by the mere disruption of either NF-κB or STAT3 signaling (Figure 1H).
IL-6, a multifunctional pro-inflammatory cytokine, is involved in cell survival, apoptosis, proliferation, immune responses, and tumorigenesis (Naugler and Karin, 2008). The role of IL-6 in carcinogenesis is especially pronounced in multiple myeloma (MM), a neoplasm of plasma cells. Renal failure is a major cause of mortality in multiple myeloma patients. Similar to previous observations (Kimura et al., 2003; Powell et al., 2005), we also observed that severe kidney failure with marked proteinuria is a primary cause of death (FigureS4E). Additional to onset of kidney failure, we demonstrated that Rrm2b−/− mice developed dysfunctional immunologic and hematopoietic systems (high serum IL-6, splenic hemorrhage, abnormal IgM and IgG levels, lymphocytosis, neutrophilia, and thrombocytosis). Furthermore, some aged Rrm2b+/− mice developed a plasmacytoma-like neoplasm, progressive splenomegaly, enlarged lymph nodes and ascites. Blockade or modulation of the IL-6 signal is a strategy that is well on its way to clinical trials for lymphoma, multiple myeloma (MM) and chronic inflammatory diseases (Naugler and Karin, 2008). Interestingly, Rrm2b−/−IL-6−/− mice lived longer than Rrm2b−/− mice by two more months (Figure 4E). Splenic hemorrhage, abnormal hematologic parameters and proteinuria were dramatically reduced in Rrm2b−/−IL-6−/− double mutants (Figure 4). Similar to their age-matched WT littermates, kidney and spleen samples from Rrm2b−/−IL-6−/− showed no pathologic findings. These results strongly suggest that IL-6 partly contributes to the development of hematologic disorders and mortality from renal failure in Rrm2b−/− mice.
Our findings demonstrate a potential role of Rrm2b in prohibiting oncogenesis. Earlier data implicated that Rrm2b protein level might be negatively correlated to cancer progression (Liu et al., 2011; Zhang et al., 2011). Based on the observed phenotypes from Rrm2b-deficient mice model, Rrm2b deficiency might be a potential risk factor for hematologic malignancy. Indeed, Rrm2b proteins were much reduced in myeloma cells of MM patients (Figure 4F). Rrm2b deletion, mutation or haploinsufficiency can serve as a diagnostic marker in MM or other lymphomas. Finally, Rrm2b reduction could potentially be used in personalized medicine to identify patients who are susceptible to the inflammatory side effects and cancer risk of traditional radiation therapy or DNA-damaging chemotherapeutic drugs.
EXPERIMENTAL PROCEDURES
Mice and Cell Culture
Generation of Rrm2b−/− mice by the gene trap method has been described (Powell et al., 2005). Rrm2b+/− mice in mixed genetic background (129/SvEvBrd and C57BL/6J) were obtained from the Texas A&M Institute for Genomic Medicine (TIGM) and backcrossed to C57BL/6J mice for 7 generations. IL-6−/− mice (stock number: 002650, C57BL/6J background) were purchased from The Jackson Laboratory. MM specimens (IRB10046) were obtained from the tissue bank of Translational Research Core (TLR) at City of Hope. Murine studies were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) of City of Hope. In vitro MEFs experiments using the mice that have been had been backcrossed to C57BL/6 for at least 7 generations, except for Rrm2b−/− fibroblasts (Figure 1F) which were derived from mouse embryos in mixed sv129 and C57BL/6 background (Tanaka et al., 2000).
Supplementary Material
Highlight.
Constitutive NF-KB and STAT3 Mediate Sustained IL-6 Expression in Rrm2b-Deficient Cells
Loss of Rrm2b Gene Causes Chromosomal Instability
Mice Deficient in Rrm2b Exhibit Elevated IL-6 and Hematologic Disorders
IL-6 Deficiency Delayed Disease Onset and Mortality of Rrm2b−/− mice
Acknowledgments
We gratefully thank Michael Karin (UCSD) for his review and comments on our manuscript, Yibin Wang (UCLA) for adenoviruses expressing IκBα mutant super-repressor (S32A, S36A), Yusuke Nakamura (The University of Tokyo) for Rrm2b−/− fibroblasts, and Yafan Wang for searching MM specimens in TLR core. Research was supported by the grants from the Lymphoma SPORE to L.C. and Y.Y., the Tim Nesvig Lymphoma Research Foundation to L.C. and Y.Y. and the National Cancer Institute (5R01CA127541) to Y.Y.
Footnotes
The supplemental information includes Extended Experimental Procedures and four Figures that can be found with this article online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bourdon A, Minai L, Serre V, Jais JP, Sarzi E, Aubert S, Chretien D, de Lonlay P, Paquis-Flucklinger V, Arakawa H, et al. Mutation of RRM2B, encoding p53-controlled ribonucleotide reductase (p53R2), causes severe mitochondrial DNA depletion. Nature genetics. 2007;39:776–780. doi: 10.1038/ng2040. [DOI] [PubMed] [Google Scholar]
- Chang L, Zhou B, Hu S, Guo R, Liu X, Jones SN, Yen Y. ATM-mediated serine 72 phosphorylation stabilizes ribonucleotide reductase small subunit p53R2 protein against MDM2 to DNA damage. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18519–18524. doi: 10.1073/pnas.0803313105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S, Karin M. Autocrine IL-6 signaling: a key event in tumorigenesis? Cancer Cell. 2008;13:7–9. doi: 10.1016/j.ccr.2007.12.020. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Takeda S, Sagiya Y, Gotoh M, Nakamura Y, Arakawa H. Impaired function of p53R2 in Rrm2b-null mice causes severe renal failure through attenuation of dNTP pools. Nature genetics. 2003;34:440–445. doi: 10.1038/ng1212. [DOI] [PubMed] [Google Scholar]
- Kumar D, Abdulovic AL, Viberg J, Nilsson AK, Kunkel TA, Chabes A. Mechanisms of mutagenesis in vivo due to imbalanced dNTP pools. Nucleic Acids Res. 2011;39:1360–1371. doi: 10.1093/nar/gkq829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Lai L, Wang X, Xue L, Leora S, Wu J, Hu S, Zhang K, Kuo ML, Zhou L, et al. Ribonucleotide reductase small subunit M2B prognoses better survival in colorectal cancer. Cancer research. 2011;71:3202–3213. doi: 10.1158/0008-5472.CAN-11-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Naugler WE, Karin M. The wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14:109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Nordlund P, Reichard P. Ribonucleotide reductases. Annu Rev Biochem. 2006;75:681–706. doi: 10.1146/annurev.biochem.75.103004.142443. [DOI] [PubMed] [Google Scholar]
- Powell DR, Desai U, Sparks MJ, Hansen G, Gay J, Schrick J, Shi ZZ, Hicks J, Vogel P. Rapid development of glomerular injury and renal failure in mice lacking p53R2. Pediatr Nephrol. 2005;20:432–440. doi: 10.1007/s00467-004-1696-5. [DOI] [PubMed] [Google Scholar]
- Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Tanaka H, Arakawa H, Yamaguchi T, Shiraishi K, Fukuda S, Matsui K, Takei Y, Nakamura Y. A ribonucleotide reductase gene involved in a p53-dependent cell-cycle checkpoint for DNA damage. Nature. 2000;404:42–49. doi: 10.1038/35003506. [DOI] [PubMed] [Google Scholar]
- Wu ZH, Shi Y, Tibbetts RS, Miyamoto S. Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science. 2006;311:1141–1146. doi: 10.1126/science.1121513. [DOI] [PubMed] [Google Scholar]
- Zhang K, Wu J, Wu X, Wang X, Wang Y, Zhou N, Kuo ML, Liu X, Zhou B, Chang L, et al. p53R2 inhibits the proliferation of human cancer cells in association with cell-cycle arrest. Mol Cancer Ther. 2011;10:269–278. doi: 10.1158/1535-7163.MCT-10-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Dai H, Zhou M, Li M, Singh P, Qiu J, Tsark W, Huang Q, Kernstine K, Zhang X, et al. Fen1 mutations result in autoimmunity, chronic inflammation and cancers. Nat Med. 2007;13:812–819. doi: 10.1038/nm1599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.