Abstract
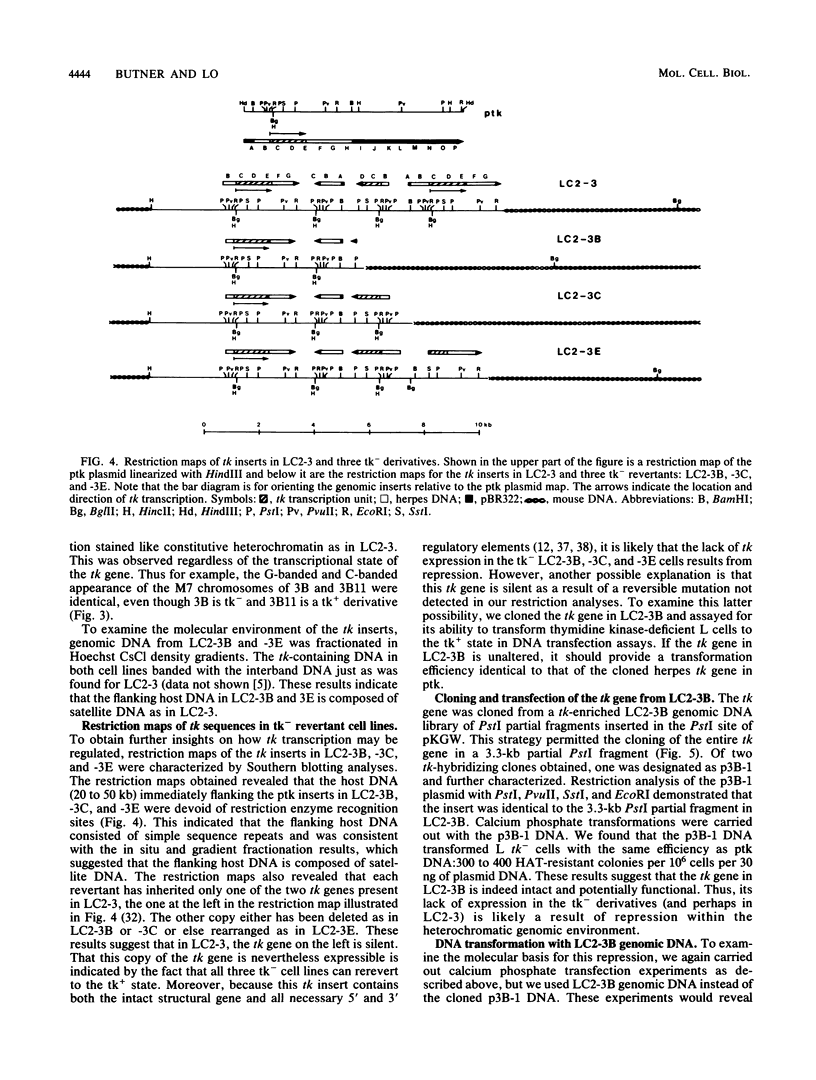
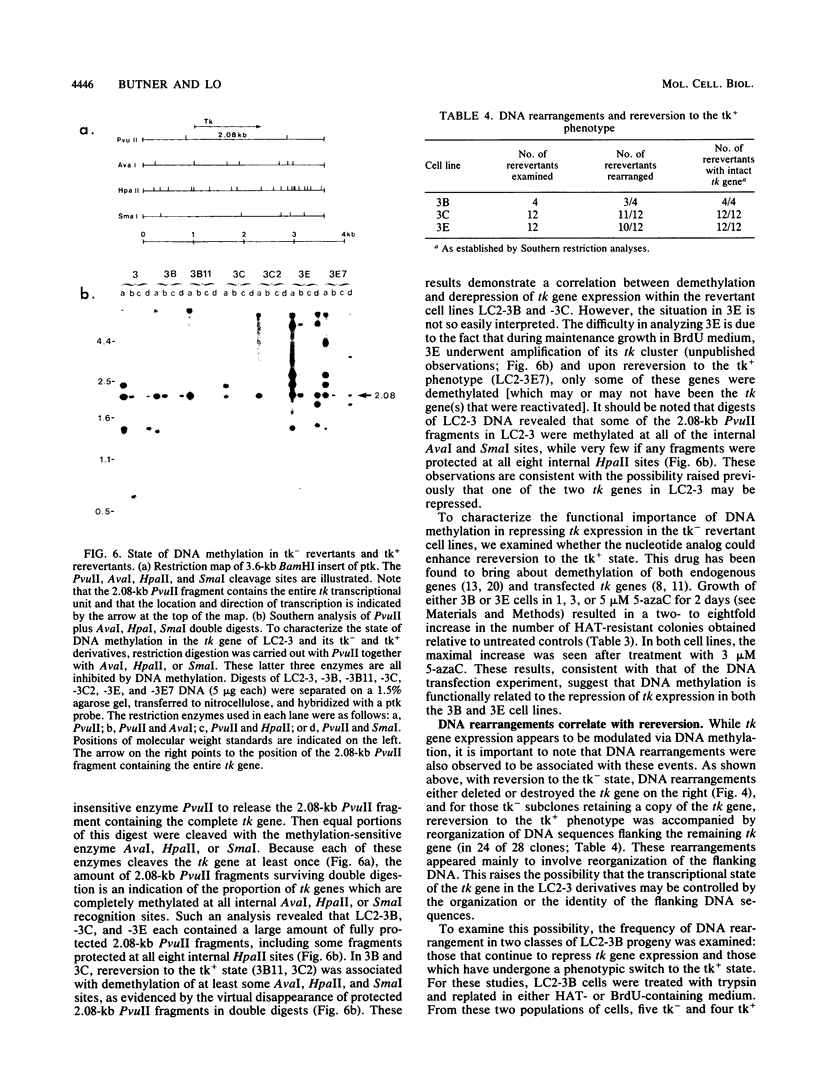
We have obtained a mouse transformant cell line containing two herpes viral thymidine kinase (tk) genes integrated in pericentromeric heterochromatin. Restriction analysis of tk- revertant and tk+ rerevertant derivatives suggest that one of the two tk genes is repressed in tk- cells, but is reactivated in tk+ rerevertants. The results of Northern analysis indicated that repression-activation is probably controlled at the transcriptional level. To examine the molecular basis for this repression, we cloned the tk gene from a tk- revertant cell line. Then, using the cloned tk gene as donor DNA to select for tk+ transformants, we found that it has a transfection efficiency indistinguishable from the viral tk gene. This indicates that repression is probably not mediated via any DNA sequence changes within the tk gene. The results of further studies by restriction analysis, azacytidine treatments, and secondary DNA transfection assays demonstrated that tk repression is associated with changes in DNA methylation. Surprisingly, derepression of the tk gene was accompanied by rearrangements in the flanking DNA. The latter result suggests that the flanking DNA may exert cis effects on tk gene expression. Additional studies with this system may provide insights into the molecular basis underlying position effects in heterochromatin.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker W. K. Position-effect variegation. Adv Genet. 1968;14:133–169. [PubMed] [Google Scholar]
- Brown S. W. Heterochromatin. Science. 1966 Jan 28;151(3709):417–425. doi: 10.1126/science.151.3709.417. [DOI] [PubMed] [Google Scholar]
- Brutlag D. L. Molecular arrangement and evolution of heterochromatic DNA. Annu Rev Genet. 1980;14:121–144. doi: 10.1146/annurev.ge.14.120180.001005. [DOI] [PubMed] [Google Scholar]
- Burgoyne P. S. Genetic homology and crossing over in the X and Y chromosomes of Mammals. Hum Genet. 1982;61(2):85–90. doi: 10.1007/BF00274192. [DOI] [PubMed] [Google Scholar]
- Butner K. A., Lo C. W. High frequency DNA rearrangements associated with mouse centromeric satellite DNA. J Mol Biol. 1986 Feb 20;187(4):547–556. doi: 10.1016/0022-2836(86)90333-5. [DOI] [PubMed] [Google Scholar]
- Cattanach B. M. Position effect variegation in the mouse. Genet Res. 1974 Jun;23(3):291–306. doi: 10.1017/s0016672300014932. [DOI] [PubMed] [Google Scholar]
- Chapman V. M., Kratzer P. G., Siracusa L. D., Quarantillo B. A., Evans R., Liskay R. M. Evidence for DNA modification in the maintenance of X-chromosome inactivation of adult mouse tissues. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5357–5361. doi: 10.1073/pnas.79.17.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy B. A., Scangos G. A. Changes in structure and methylation pattern in a cluster of thymidine kinase genes. Mol Cell Biol. 1984 Apr;4(4):611–617. doi: 10.1128/mcb.4.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy B., Scangos G. Expression of transferred thymidine kinase genes is controlled by methylation. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6299–6303. doi: 10.1073/pnas.79.20.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough D. W., Kunkel L. M., Davidson R. L. 5-Azacytidine-induced reactivation of a herpes simplex thymidine kinase gene. Science. 1982 Apr 2;216(4541):70–73. doi: 10.1126/science.6175023. [DOI] [PubMed] [Google Scholar]
- Colbere-Garapin F., Chousterman S., Horodniceanu F., Kourilsky P., Garapin A. C. Cloning of the active thymidine kinase gene of herpes simplex virus type 1 in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3755–3759. doi: 10.1073/pnas.76.8.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compere S. J., Palmiter R. D. DNA methylation controls the inducibility of the mouse metallothionein-I gene lymphoid cells. Cell. 1981 Jul;25(1):233–240. doi: 10.1016/0092-8674(81)90248-8. [DOI] [PubMed] [Google Scholar]
- Davies R. L., Fuhrer-Krusi S., Kucherlapati R. S. Modulation of transfected gene expression mediated by changes in chromatin structure. Cell. 1982 Dec;31(3 Pt 2):521–529. doi: 10.1016/0092-8674(82)90308-7. [DOI] [PubMed] [Google Scholar]
- Davis R. W., Thomas M., Cameron J., St John T. P., Scherer S., Padgett R. A. Rapid DNA isolations for enzymatic and hybridization analysis. Methods Enzymol. 1980;65(1):404–411. doi: 10.1016/s0076-6879(80)65051-4. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Francke U., Oliver N. Quantitative analysis of high-resolution trypsin-giemsa bands on human prometaphase chromosomes. Hum Genet. 1978 Dec 18;45(2):137–165. doi: 10.1007/BF00286957. [DOI] [PubMed] [Google Scholar]
- Gartler S. M., Andina R. J. Mammalian X-chromosome inactivation. Adv Hum Genet. 1976;7:99–140. doi: 10.1007/978-1-4757-0659-8_3. [DOI] [PubMed] [Google Scholar]
- Gartler S. M., Riggs A. D. Mammalian X-chromosome inactivation. Annu Rev Genet. 1983;17:155–190. doi: 10.1146/annurev.ge.17.120183.001103. [DOI] [PubMed] [Google Scholar]
- Groudine M., Eisenman R., Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981 Jul 23;292(5821):311–317. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- Hardies S. C., Axelrod D. E., Edgell M. H., Hutchison C. A., 3rd Phenotypic variation associated with molecular alterations at a cluster of thymidine kinase genes. Mol Cell Biol. 1983 Jul;3(7):1163–1171. doi: 10.1128/mcb.3.7.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper M. E., Saunders G. F. Localization of single copy DNA sequences of G-banded human chromosomes by in situ hybridization. Chromosoma. 1981;83(3):431–439. doi: 10.1007/BF00327364. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Position-effect variegation and chromosome structure of a heat shock puff in Drosophila. Chromosoma. 1981;83(3):381–393. doi: 10.1007/BF00327360. [DOI] [PubMed] [Google Scholar]
- Hilliker A. J., Appels R., Schalet A. The genetic analysis of D. melanogaster heterochromatin. Cell. 1980 Oct;21(3):607–619. doi: 10.1016/0092-8674(80)90424-9. [DOI] [PubMed] [Google Scholar]
- John B., Miklos G. L. Functional aspects of satellite DNA and heterochromatin. Int Rev Cytol. 1979;58:1–114. doi: 10.1016/s0074-7696(08)61473-4. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M., Mohandas T., Shapiro L. J. Cell cycle-specific reactivation of an inactive X-chromosome locus by 5-azadeoxycytidine. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1215–1219. doi: 10.1073/pnas.79.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS E. B. The phenomenon of position effect. Adv Genet. 1950;3:73–115. doi: 10.1016/s0065-2660(08)60083-8. [DOI] [PubMed] [Google Scholar]
- Lewis W. H., Srinivasan P. R., Stokoe N., Siminovitch L. Parameters governing the transfer of the genes for thymidine kinase and dihydrofolate reductase into mouse cells using metaphase chromosomes or DNA. Somatic Cell Genet. 1980 May;6(3):333–347. doi: 10.1007/BF01542787. [DOI] [PubMed] [Google Scholar]
- Liskay R. M., Evans R. J. Inactive X chromosome DNA does not function in DNA-mediated cell transformation for the hypoxanthine phosphoribosyltransferase gene. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4895–4898. doi: 10.1073/pnas.77.8.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C. W. Novel approach for restriction mapping repetitive DNA elements using DNA transformation. Somat Cell Mol Genet. 1985 Sep;11(5):455–465. doi: 10.1007/BF01534839. [DOI] [PubMed] [Google Scholar]
- Lo C. W. Transformation by iontophoretic microinjection of DNA: multiple integrations without tandem insertions. Mol Cell Biol. 1983 Oct;3(10):1803–1814. doi: 10.1128/mcb.3.10.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon M. F. X-chromosome inactivation and developmental patterns in mammals. Biol Rev Camb Philos Soc. 1972 Jan;47(1):1–35. doi: 10.1111/j.1469-185x.1972.tb00969.x. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L. A simplified method for preparation of mouse satellite DNA. Anal Biochem. 1977 Apr;78(2):561–568. doi: 10.1016/0003-2697(77)90118-x. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R. Expression of the herpes thymidine kinase gene in Xenopus laevis oocytes: an assay for the study of deletion mutants constructed in vitro. Nucleic Acids Res. 1980 Dec 20;8(24):5931–5948. doi: 10.1093/nar/8.24.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R., Kingsbury R., Axel R. Analysis of transcriptional regulatory signals of the HSV thymidine kinase gene: identification of an upstream control region. Cell. 1981 Aug;25(2):385–398. doi: 10.1016/0092-8674(81)90057-x. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Ostrander M., Vogel S., Silverstein S. Phenotypic switching in cells transformed with the herpes simplex virus thymidine kinase gene. Mol Cell Biol. 1982 Jun;2(6):708–714. doi: 10.1128/mcb.2.6.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polani P. E. Pairing of X and Y chromosomes, non-inactivation of X-linked genes, and the maleness factor. Hum Genet. 1982;60(3):207–211. doi: 10.1007/BF00303003. [DOI] [PubMed] [Google Scholar]
- Pollack Y., Stein R., Razin A., Cedar H. Methylation of foreign DNA sequences in eukaryotic cells. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6463–6467. doi: 10.1073/pnas.77.11.6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Cedar H. DNA methylation in eukaryotic cells. Int Rev Cytol. 1984;92:159–185. doi: 10.1016/s0074-7696(08)61327-3. [DOI] [PubMed] [Google Scholar]
- Roginski R. S., Skoultchi A. I., Henthorn P., Smithies O., Hsiung N., Kucherlapati R. Coordinate modulation of transfected HSV thymidine kinase and human globin genes. Cell. 1983 Nov;35(1):149–155. doi: 10.1016/0092-8674(83)90217-9. [DOI] [PubMed] [Google Scholar]
- Rushlow C. A., Bender W., Chovnick A. Studies on the mechanism of heterochromatic position effect at the rosy locus of Drosophila melanogaster. Genetics. 1984 Nov;108(3):603–615. doi: 10.1093/genetics/108.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M. F. Highly repeated sequences in mammalian genomes. Int Rev Cytol. 1982;76:67–112. doi: 10.1016/s0074-7696(08)61789-1. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Sumner A. T. A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res. 1972 Nov;75(1):304–306. doi: 10.1016/0014-4827(72)90558-7. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urieli-Shoval S., Gruenbaum Y., Sedat J., Razin A. The absence of detectable methylated bases in Drosophila melanogaster DNA. FEBS Lett. 1982 Sep 6;146(1):148–152. doi: 10.1016/0014-5793(82)80723-0. [DOI] [PubMed] [Google Scholar]
- Venolia L., Gartler S. M. Comparison of transformation efficiency of human active and inactive X-chromosomal DNA. Nature. 1983 Mar 3;302(5903):82–83. doi: 10.1038/302082a0. [DOI] [PubMed] [Google Scholar]
- Venolia L., Gartler S. M., Wassman E. R., Yen P., Mohandas T., Shapiro L. J. Transformation with DNA from 5-azacytidine-reactivated X chromosomes. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2352–2354. doi: 10.1073/pnas.79.7.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake C. T., Gudewicz T., Porter T., White A., Wilson J. H. How damaged is the biologically active subpopulation of transfected DNA? Mol Cell Biol. 1984 Mar;4(3):387–398. doi: 10.1128/mcb.4.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Levy D., Perucho M. The somatic replication of DNA methylation. Cell. 1981 Apr;24(1):33–40. doi: 10.1016/0092-8674(81)90498-0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Yen P. H., Patel P., Chinault A. C., Mohandas T., Shapiro L. J. Differential methylation of hypoxanthine phosphoribosyltransferase genes on active and inactive human X chromosomes. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1759–1763. doi: 10.1073/pnas.81.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]