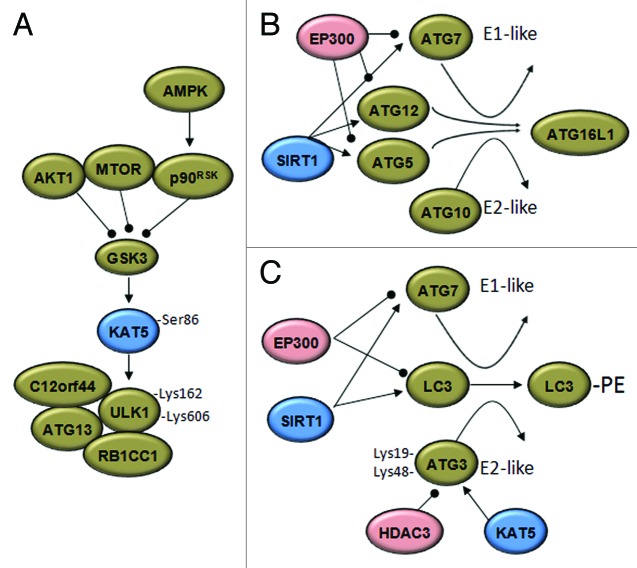
Figure 2. Acetylases and deacetylases modify the autophagic machinery. (A) Under growth factor deprivation, the activation of glycogen synthase kinase-3 (GSK3) during autophagy initiation results in phosphorylation of the KAT5 acetyltransferase, which activates the ULK1 kinase. p90RSK, RPS6K p90. (B) The EP300 acetyltransferase inhibits the elongation of the autopahosome membranes by acetylating ATG5, ATG7, ATG8 and ATG12. Under starved conditions, SIRT1 deacetylates these ATG proteins and induces autophagy. (C) Results in yeast support the idea that starvation-induced acetylation of Atg3 at K19 and K48 by Esa1 (KAT5) controls its interaction with Atg8 (LC3) and Atg8 lipidation. Deacetylation of Atg3 is accomplished by the deacetylase Rpd3 (HDAC3). Arrowheads and balls respectively indicate inducing or inhibitory effects.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.