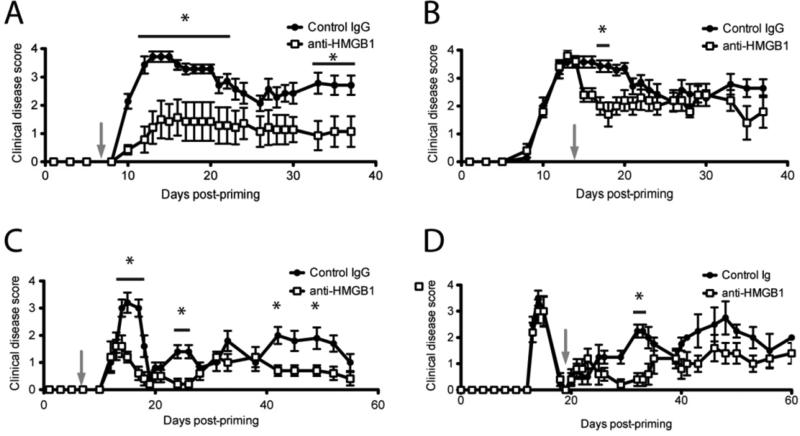
Figure 2. HMGB1 contributes to EAE pathogenesis.
For C-EAE, C57BL/6 mice, 6-8 wks of age, were immunized with 200 μg MOG35–55/CFA s.c., received 200 ng pertussis toxin i.p. on days 0 and 2, and scored daily for clinical disease using a standard five-point scale. C-EAE mice were injected i.v. with 100 μg anti-HMGB1 neutralizing antibody or isotype control antibody in PBS 7 days (A) or 14 days (B) after disease induction (grey arrows). For R-EAE, SJL/J mice, 6-8 wks of age, were immunized with 50 μg PLP139-151/CFA s.c. and scored daily for clinical disease using a standard five-point scale. R-EAE mice were injected i.v. with 100 μg anti-HMGB1 neutralizing antibody or isotype control antibody 7 days after disease induction (C) or at disease remission, 19 days after induction (D) (grey arrows). Data are representative of two experiments of ≥ 5 mice per group. Main effects of treatment were tested for by two-way ANOVA. Asterisks denote a significant difference in mean clinical score for the indicated days by Tukey's post hoc tests (p < 0.05).