Fig 1. Conceptual Framework of Translating Biomarkers of Treatment Response to Develop Rapid Acting Antidepressants.
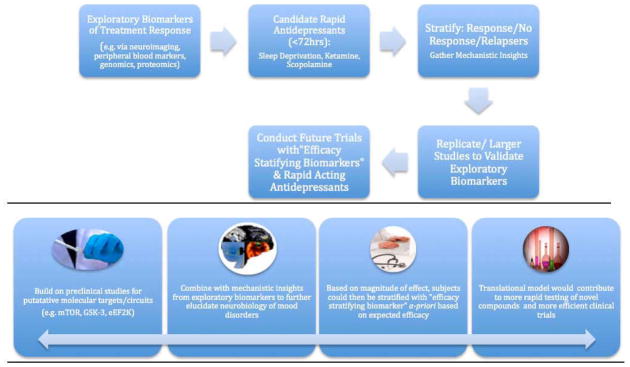
This model characterizes our approach to the study of biomarkers and is designed to enhance our ability to rapidly identify and test surrogate clinical and biological endpoints that predict treatment outcomes (e.g. ≥50% antidepressant response) in mood disorders. The method uses novel agents or other interventions that produce rapid antidepressant clinical effects (i.e. ≤72 hours). Results lead to both prompt evaluation of efficacy and potential insights into relevant neural and molecular correlates of outcome (e.g. mTOR activation, eEF2K inhibition). The benefits of developing biomarkers within this model are contrasted with the model used to evaluate conventional antidepressant treatments (Table S2 in Supplement 1). The long-term goal of this model is to validate successful biomarkers of response, where patients can then be stratified a priori based on expected efficacy. Overall, this will contribute to more efficient prospective trials and lead to the identification of enriched biological subgroups.
In this figure, ketamine can be used as a prototype: approximately 50% of subjects achieve response within four hours thus permitting the assessment of multiple biomarkers (e.g. fMRI, MEG, PSG, BDNF, SNPs) and resulting neural and molecular correlates of outcome (Table 3). Ketamine’s antidepressant effects in most patients with treatment-resistant depression appear to fade in one to two weeks. Thus, this time point also permits the opportunity to assess the molecular and neural correlates of relapse in mood disorders.
