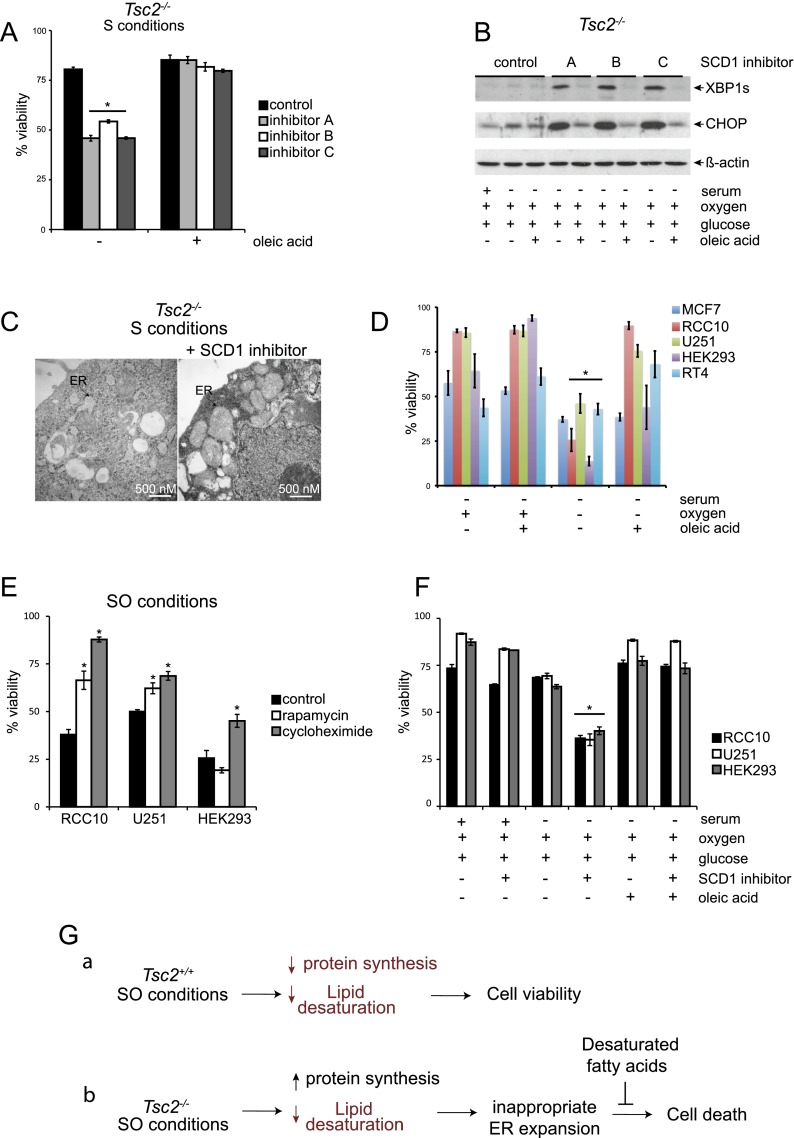
Figure 7.
SCD1 inhibition links hypoxic Tsc2−/− cell death to reduced levels of unsaturated lipids under low O2. (A) To investigate the link between hypoxic Tsc2−/− cell death and lipid desaturation and test whether oleic acid could reverse cell death, Tsc2−/−, p53−/− MEFs were cultured under S conditions with or without three different SCD1 inhibitors (inhibitor A: Cayman Chemical CAY10566, used at 15 nM; inhibitor B: BioVision, used at 3 μM; and inhibitor C: Selleck Chemicals MK-8245, used at 3 μM) and with or without 50 μM oleic acid (P < 0.001). (B) To determine whether inhibition of SCD1 in Tsc2-null cells alters UPR signaling pathways, Tsc2−/−, p53−/− MEFs were cultured under S conditions with or without the SCD1 inhibitors A, B, and C described above and oleic acid. Whole-cell extracts were blotted for XBP1S, CHOP, and β-actin. (C) Representative electron micrographs for Tsc2−/−, p53−/− MEFs cultured under S conditions in the presence and absence of SCD1 inhibitor A are displayed. Black arrows highlight the ER. (D) The viability of human MCF7, RCC10, U251, HEK293, and RT4 cancer cell lines cultured under S or SO conditions in the presence or absence of oleic acid was determined by flow cytometry (P < 0.01). (E) The viability of human RCC10, U251, and HEK293 cells after 20 nM rapamycin and 1 μM cycloheximide treatment was examined after 72 h of exposure to SO conditions (P < 0.001). (F) The viability of human RCC10, U251, and HEK293 cells after treatment with SCD1 inhibitor A (Cayman Chemicals) at 1 μM cultured under replete, serum-deprived, and serum- and oxygen-deprived conditions for 72 h with or without treatment with 50 μM oleic acid was examined by flow cytometry (P < 0.001). (G) Model comparing how SO limitation affects stress signaling pathways and viability in Tsc2+/+, p53−/− and Tsc2−/−, p53−/− MEFs. Under SO conditions, levels of desaturated lipids are reduced in Tsc2+/+, p53−/− MEFs; however, this does not affect cell viability because these cells appropriately down-regulate mTORC1 activity. In contrast, we suggest that Tsc2−/−, p53−/− MEFs exhibit elevated protein synthesis with an increased load of unfolded proteins as well as reduced levels of desaturated lipids under SO conditions. This results in a magnified UPR, which cannot be resolved because the ER is unable to expand appropriately to resolve the increased level of unfolded proteins, leading to loss of cell viability. Addition of unsaturated fatty acids restores the ultrastructure of the ER, dampens the UPR, and rescues cell viability. Pathways or components of the model that are down-regulated are diagrammed in red.