Figure 2.
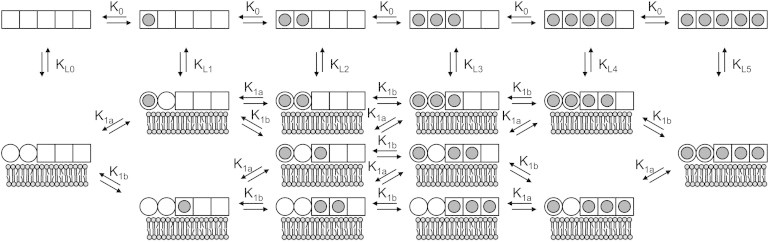
Schematic of all potential species involved in the model for binding of Ca2+ and membrane to annexin. The model is analogous to the MWC model of O2 binding hemoglobin (48), where the MWC states tensed (T) and relaxed (R) are replaced by solution and membrane-bound states. In the absence of membrane, all five Ca2+ sites are low affinity (K0) and exothermic (represented as squares). In the presence of membrane, the five Ca2+-binding sites differentiate into two classes: two high-affinity (K1a) endothermic Ca2+ sites (represented as circles) and three low-affinity (K1b) exothermic Ca2+ sites (represented as squares). Transitioning to the membrane-bound state in the absence of Ca2+ is dictated by the affinity KL, which is analogous to the equilibrium constant describing the transition between the T and R states in the MWC model. As in the classic MWC model, in each state (solution or membrane-bound), binding sites are considered to be independent and equivalent within each class.
