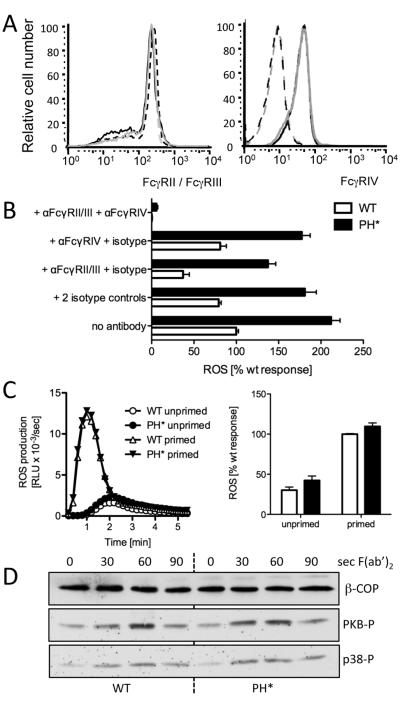
Figure 5. FcγR are not affected in Arap3PH*/PH* neutrophils.
(A) Surface distribution of FcγRII/III and FcγRIV was analysed by FACS analysis of bone marrow cell populations. Seven wild-type and seven knock-in chimeras obtained from three different pairs of donors were analysed in two independent experiments. A representative example is shown. Black lines represent control cells and grey lines Arap3PH*/PH*. FcγRII/III FACS: broken lines represent unstimulated and full lines TNFα-stimulated samples; FcγRIV FACS: dotted lines represent isotype control. (B) Immune complex induced ROS formation depends on signalling through FcγRs. Neutrophils were or were not pre-incubating with FcγR blocking antibodies and/or isotype controls as indicated, before being plated onto immobilised immune complexes for ROS assays. Pooled results from three independent experiments are plotted (mean ± SEM). Differences between wild-type and Arap3PH*/PH* were highly significant (p<0.001) for all conditions except anti-FcγRII/III + anti-FcγRIV (p>0.05), two-way ANOVA with Bonferroni post-test. (C) Cross-linking FcγRII/III does not cause increased ROS production in Arap3PH*/PH* neutrophils. Bone marrow derived neutrophils were prepared from control and knock-in mice and incubated with anti FcγRII/III with or without priming. After washing, ROS production was measured on cross-linking the FcγR with a F(ab’)2 fragment. A representative example is shown (left) and integrated results from five independent experiments are plotted (mean ± SEM). Differences did not reach statistical significance (p>0.1; Mann Whitney T-test). (D) Control and Arap3PH*/PH* neutrophils were incubated with FcγRII/III antibody, washed, and stimulated by F(ab’)2 fragment-mediated cross-linking for the indicated amounts of time. Time 0, no cross-linking. Lysates were prepared and protein subjected to immunoblotting. PKB and p38 phosphorylation was analysed using phospho-specific antibodies; β-COP served as loading control. A representative experiment from four independent experiments is shown.