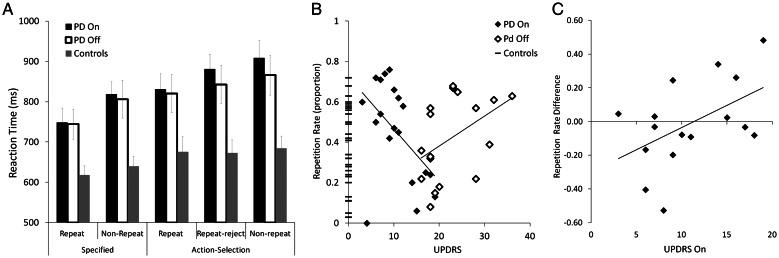
Figure 2.
(A) Reaction times for repeat, repeat-reject and non-repeat trials in the specified and action-selection conditions for patients and controls. Patient reaction times are slower than for controls, and their repeat trials are faster than the trials in which an alternative response was selected. (B) The proportion of repetitive responses (in trials where a repetition was available). At chance, repetitions should be ∼0.33. When “on” dopaminergic medication, patients with less disease severity (lower UPDRS) made more repetitions and this decreased with advancing disease. In contrast, patients “off” medication show a trend of increasing repetitions with increasing disease severity. (C) The difference in patients' repetition rates between sessions (proportion of repetitions when “off” minus proportion when “on”) as a function of disease severity, demonstrating the change in repetition when patients are withdrawn from medication. Patients who have lower UPDRS scores when “on” have a larger reduction in repetition rates when withdrawn from their medication. As UPDRS increases the difference in repetition rates between sessions decreases. At approximately an UPDRS “on” score of 11 points, the change in repetition rates starts to increase, such that those patients who have high UPDRS when “on” have increased repetition rates when withdrawn from medication.