Abstract
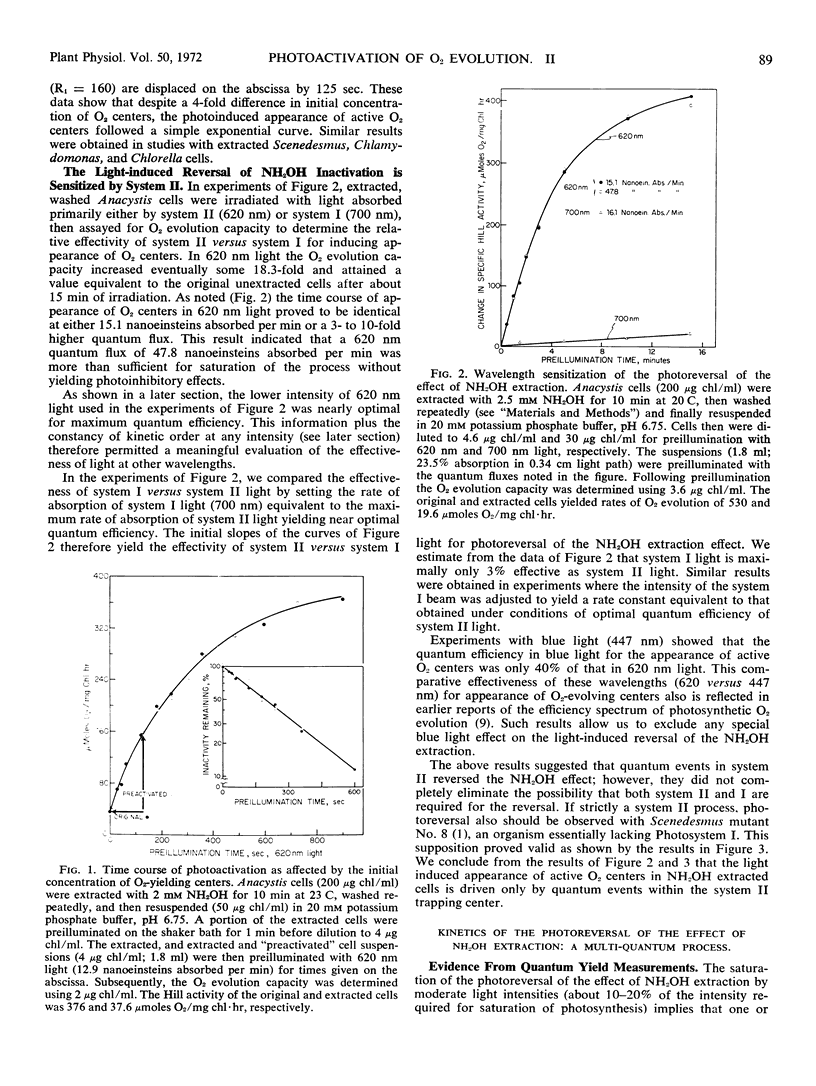
The inactivation of O2-evolving centers by NH2OH extraction was shown to be reversible. This reversal required light and manganese. This light-induced restoration of active O2-evolving centers was analyzed using three green algae and the blue-green alga, Anacystis nidulans. The following results were obtained: [List: see text]
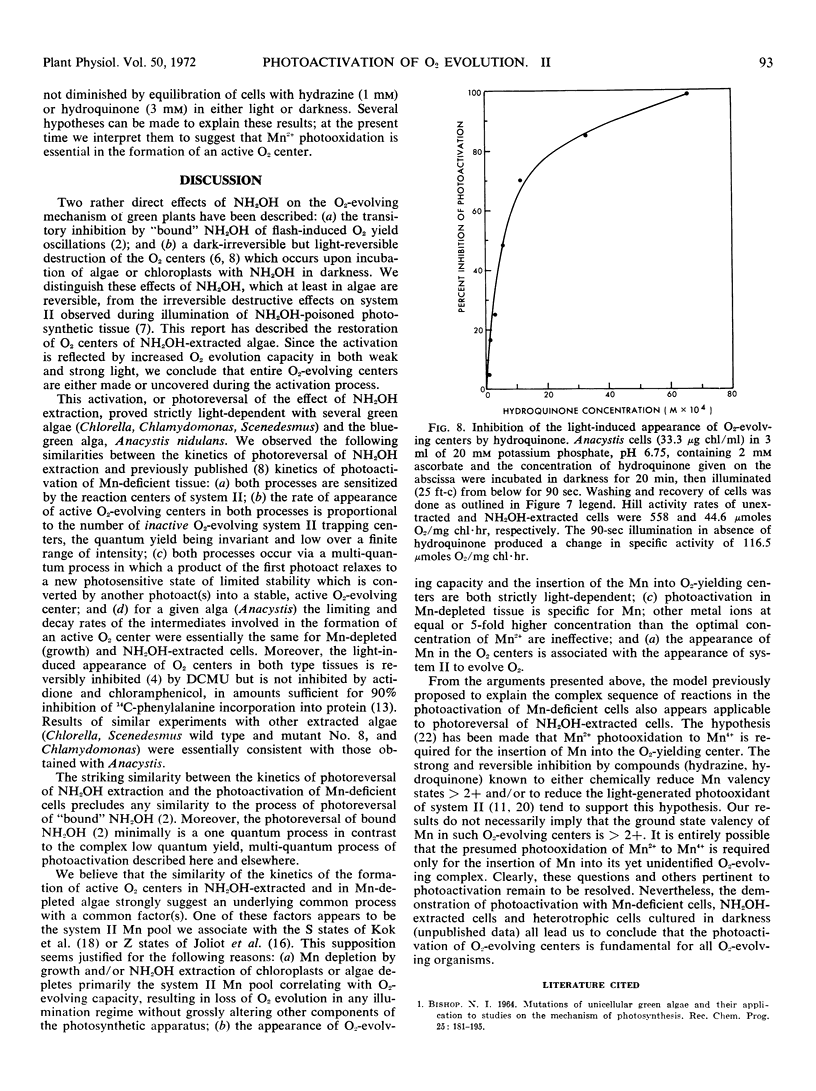
Full text
PDF

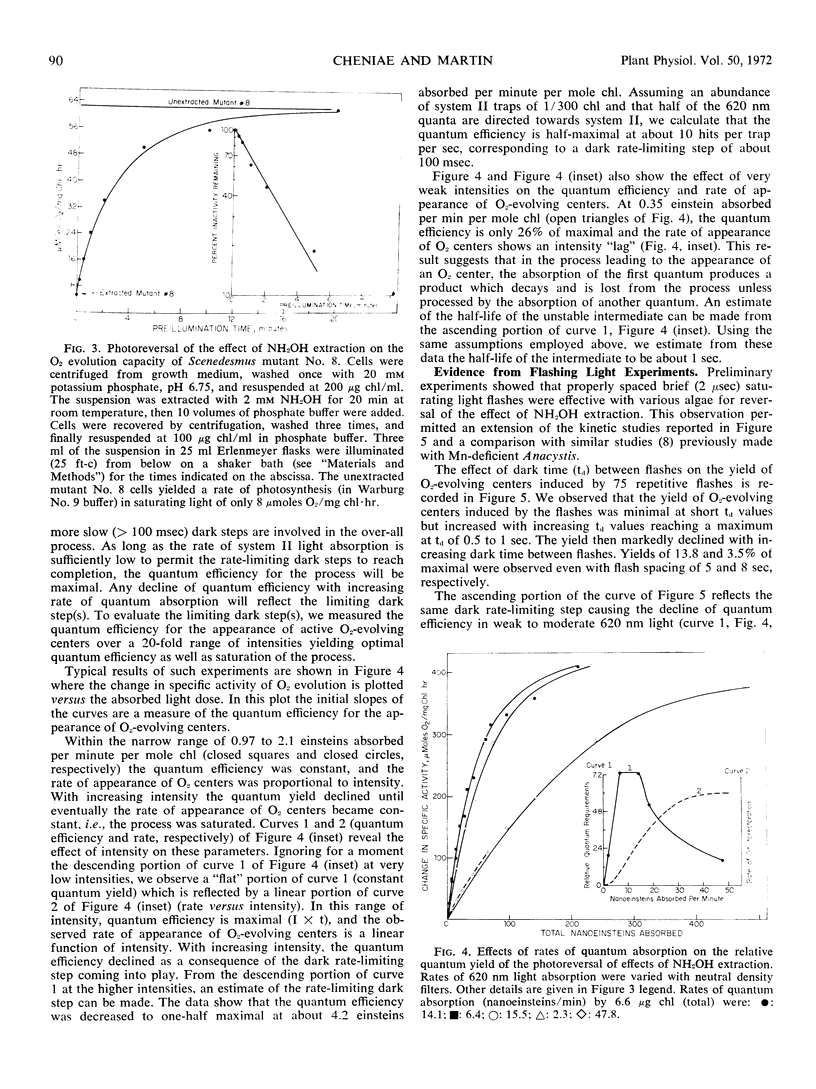

Selected References
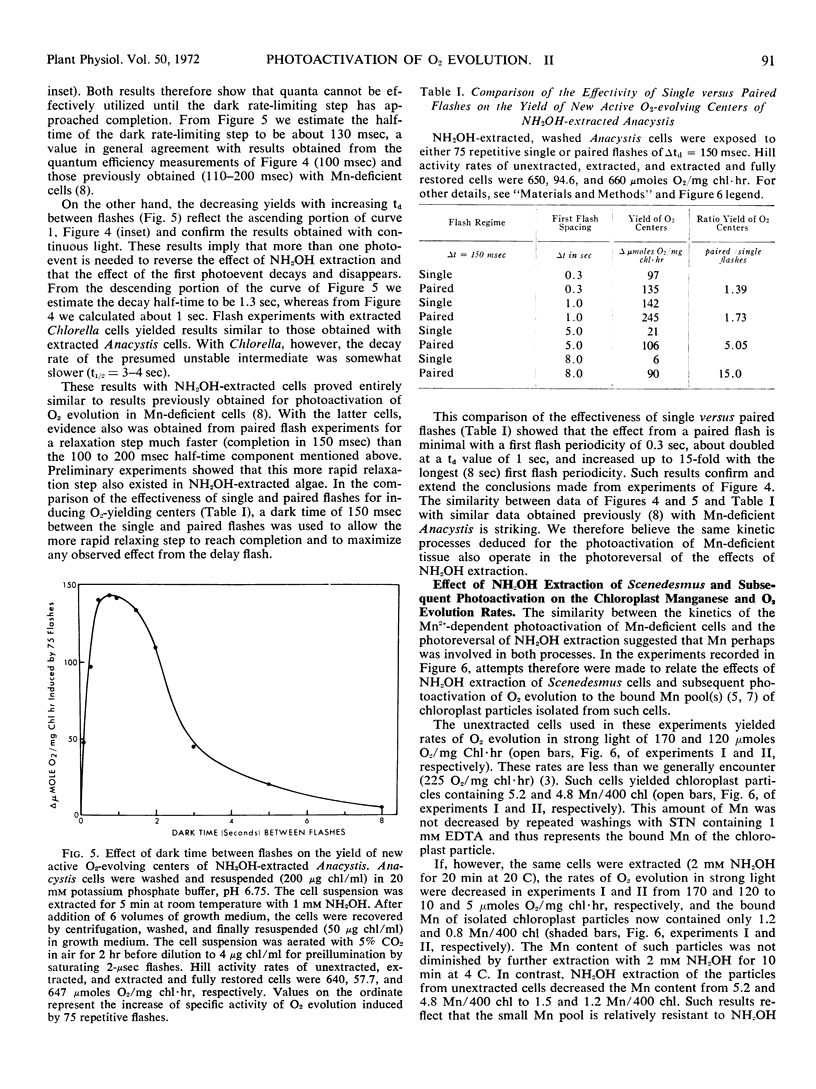
These references are in PubMed. This may not be the complete list of references from this article.
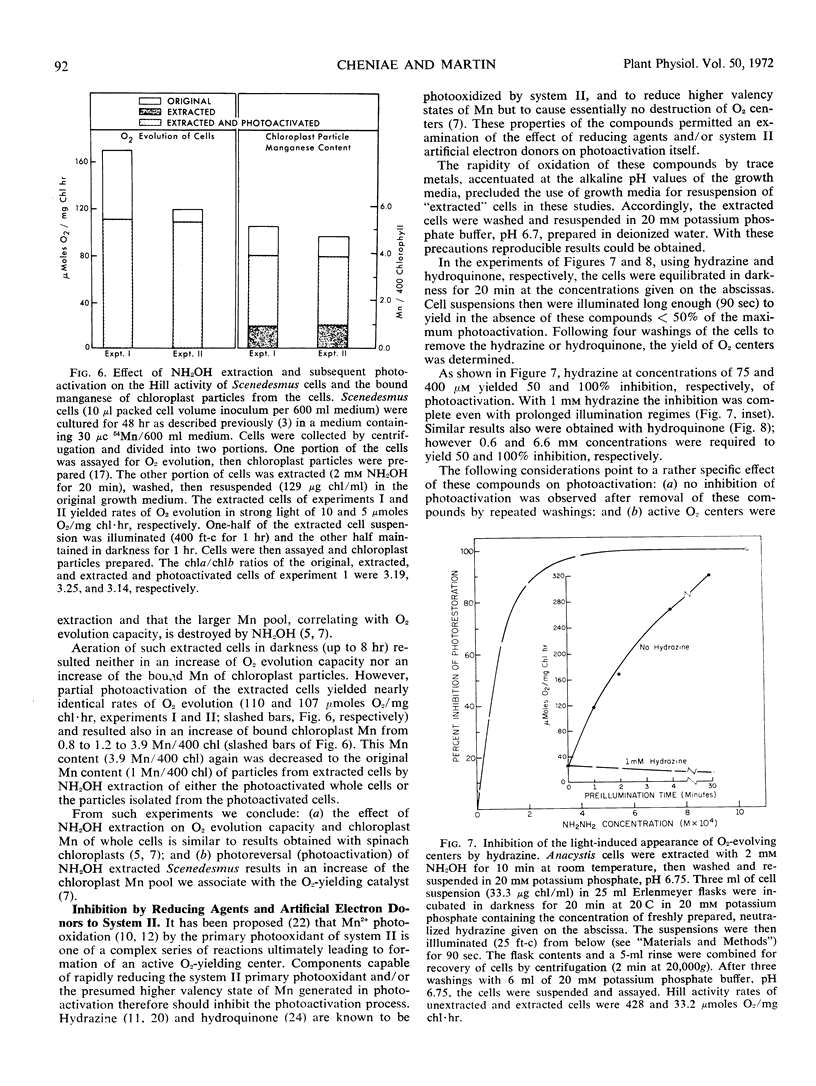
- BISHOP N. I. MUTATIONS OF UNICELLULAR GREEN ALGAE AND THEIR APPLICATION TO STUDIES ON THE MECHANISM OF PHOTOSYNTHESIS. Rec Chem Prog. 1964 Sep;25:181–195. [PubMed] [Google Scholar]
- Bouges B. Action de faibles concentrations d'hydroxylamine sur l'émission d'oxygène des algues Chlorella et des chloroplastes d'épinards. Biochim Biophys Acta. 1971 Apr 6;234(1):103–112. doi: 10.1016/0005-2728(71)90135-6. [DOI] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Effects of Hydroxylamine on Photosystem II: I. Factors Affecting the Decay of O(2) Evolution. Plant Physiol. 1971 Apr;47(4):568–575. doi: 10.1104/pp.47.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Photoactivation of the manganese catalyst of O 2 evolution. I. Biochemical and kinetic aspects. Biochim Biophys Acta. 1971 Nov 2;253(1):167–181. doi: 10.1016/0005-2728(71)90242-8. [DOI] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Photoreaction of manganese catalyst in photosynthetic oxygen evolution. Plant Physiol. 1969 Mar;44(3):351–360. doi: 10.1104/pp.44.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Site of manganese function in photosynthesis. Biochim Biophys Acta. 1968 May 28;153(4):819–837. doi: 10.1016/0005-2728(68)90009-1. [DOI] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Sites of function of manganese within photosystem II. Roles in O2 evolution and system II. Biochim Biophys Acta. 1970 Mar 3;197(2):219–239. doi: 10.1016/0005-2728(70)90033-2. [DOI] [PubMed] [Google Scholar]
- Habermann H. M., Handel M. A., McKellar P. Kinetics of chloroplast-mediated photoxidation of diketogluonate. Photochem Photobiol. 1968 Feb;7(2):211–224. doi: 10.1111/j.1751-1097.1968.tb08007.x. [DOI] [PubMed] [Google Scholar]
- Heath R. L. Hydrazine as an electron donor to the water-oxidation site in photosynthesis. Biochim Biophys Acta. 1971 Aug 6;245(1):160–164. doi: 10.1016/0005-2728(71)90018-1. [DOI] [PubMed] [Google Scholar]
- Hoober J. K., Siekevitz P., Palade G. E. Formation of chloroplast membranes in Chlamydomonas reinhardi y-1. Effects of inhibitors of protein synthesis. J Biol Chem. 1969 May 25;244(10):2621–2631. [PubMed] [Google Scholar]
- Izawa S., Heath R. L., Hind G. The role of chloride ion in photosynthesis. 3. The effect of artificial electron donors upon electron transport. Biochim Biophys Acta. 1969 Jun 24;180(2):388–398. doi: 10.1016/0005-2728(69)90123-6. [DOI] [PubMed] [Google Scholar]
- JOLIOT A., JOLIOT P. ETUDE CIN'ETIQUE DE LA R'EACTION PHOTOCHIMIQUE LIB'ERANT L'OXYG'ENE AU COURS DE LA PHOTOSYNTH'ESE. C R Hebd Seances Acad Sci. 1964 May 4;258:4622–4625. [PubMed] [Google Scholar]
- Kok B., Datko E. A. Reducing power generated in the second photoact of photosynthesis. Plant Physiol. 1965 Nov;40(6):1171–1177. doi: 10.1104/pp.40.6.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok B., Forbush B., McGloin M. Cooperation of charges in photosynthetic O2 evolution-I. A linear four step mechanism. Photochem Photobiol. 1970 Jun;11(6):457–475. doi: 10.1111/j.1751-1097.1970.tb06017.x. [DOI] [PubMed] [Google Scholar]
- Myers J., Graham J. R. The photosynthetic unit in chlorella measured by repetitive short flashes. Plant Physiol. 1971 Sep;48(3):282–286. doi: 10.1104/pp.48.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmer R., Cheniae G. M. Photoactivation of the manganese catalyst of 0 2 evolution II. A two-guantum mechanism. Biochim Biophys Acta. 1971 Nov 2;253(1):182–186. doi: 10.1016/0005-2728(71)90243-x. [DOI] [PubMed] [Google Scholar]


