Abstract
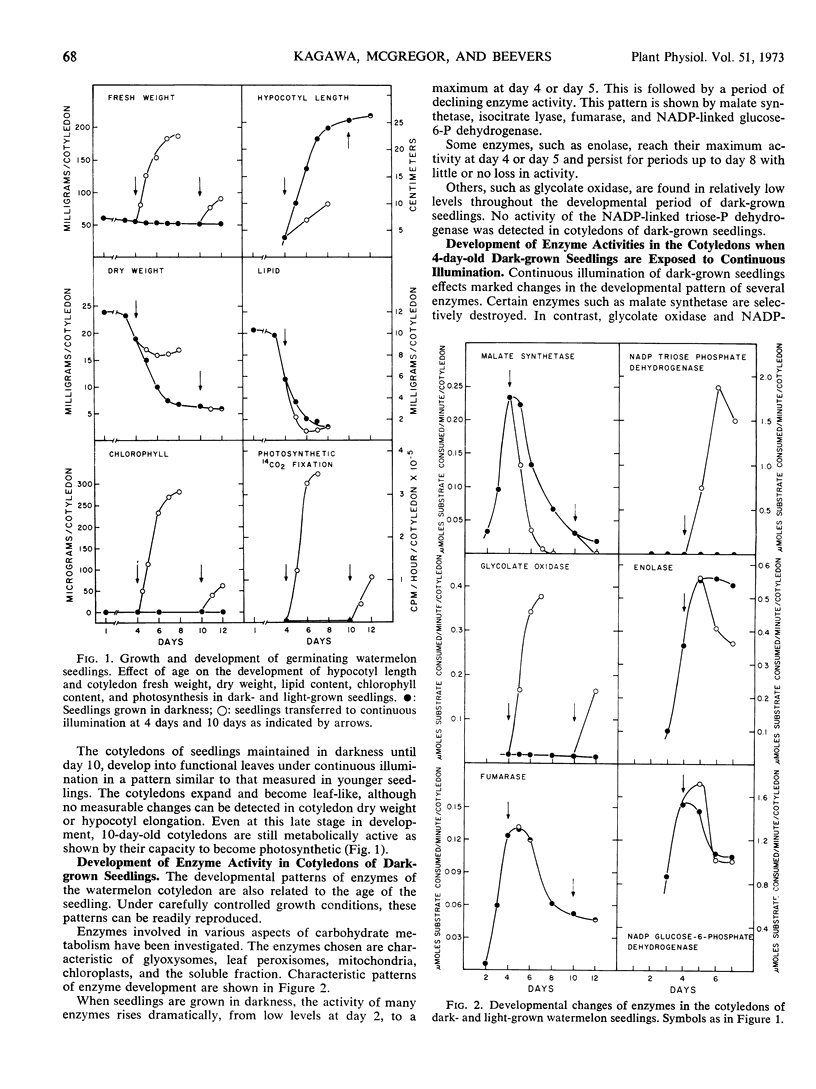
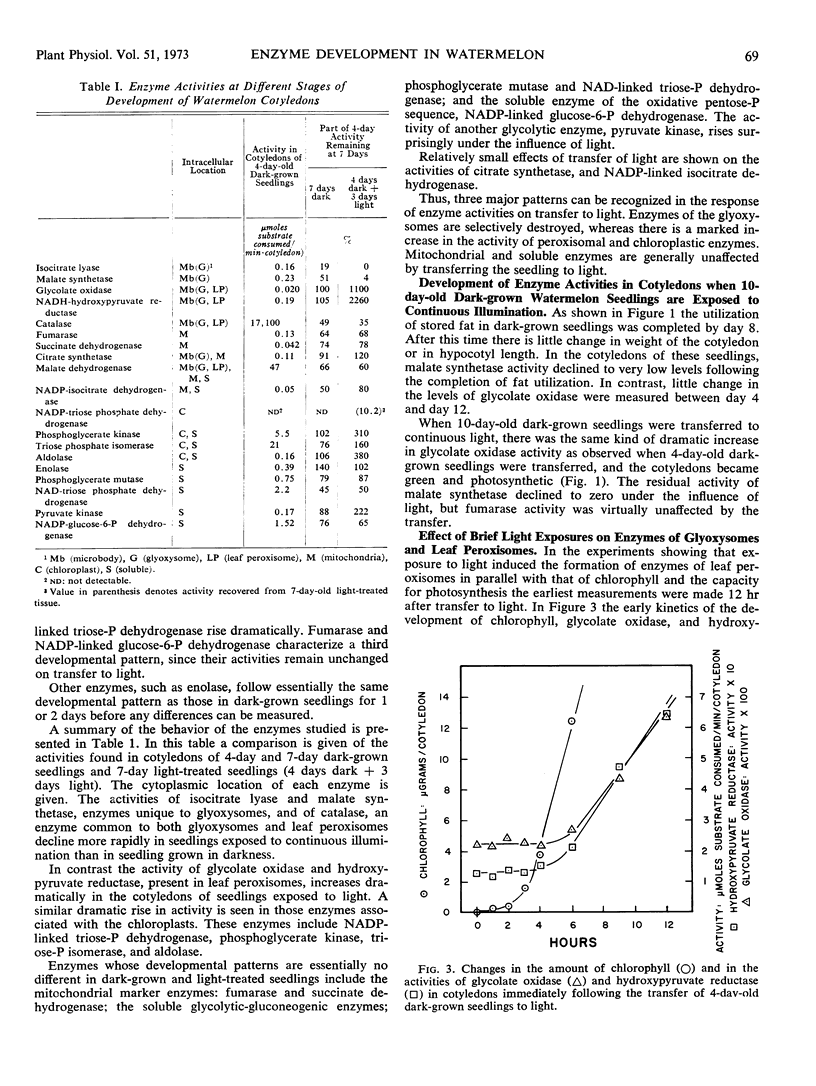
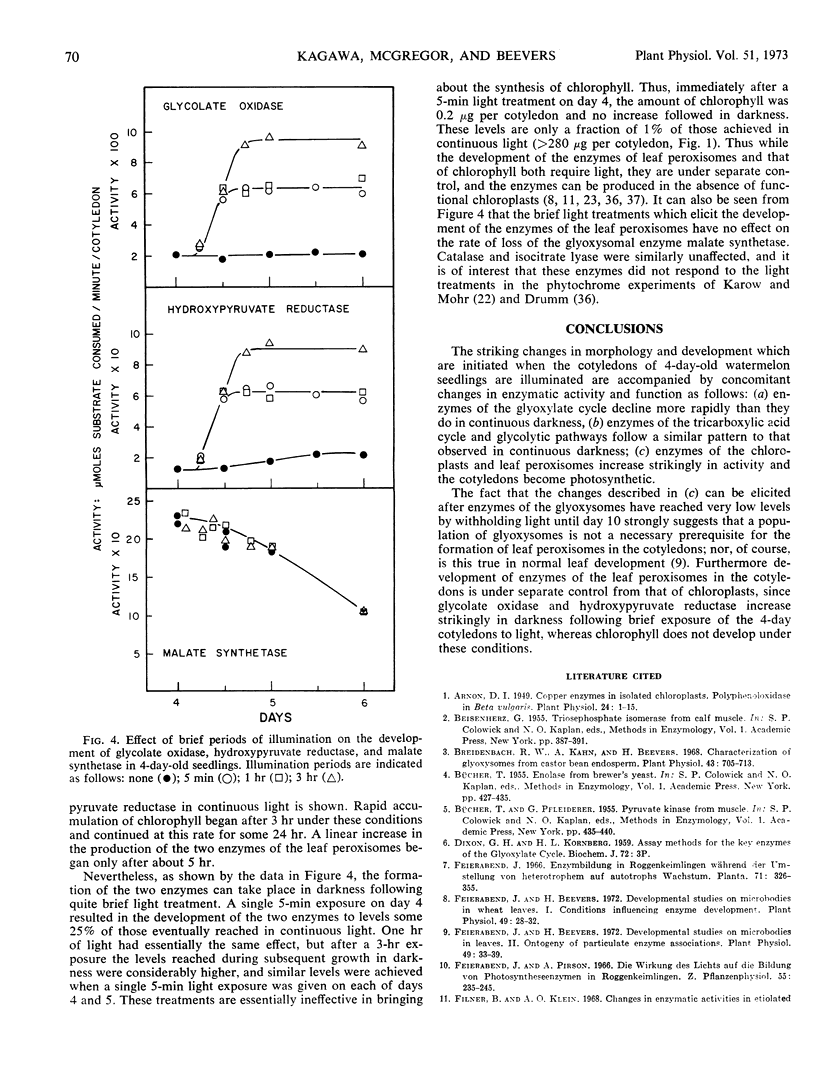
Changes in hypocotyl length, cotyledon weight, lipid content, chlorophyll content, and capacity for photosynthesis have been described in seedlings of Citrullus vulgaris, Schrad. (watermelon) growing at 30 C under various light treatments. Corresponding changes in the levels of 19 enzymes in the cotyledons are described, with particular emphasis on enzymes of microbodies, since during normal greening, enzymes of the glyoxysomes are lost and those of leaf peroxisomes appear. In complete darkness enzymes of the glyoxysomes reach a peak at 4 days and decline as the fat is depleted. Enzymes of mitochondria and of glycolytic pathways also peak at 4 to 5 days and either remain unchanged or decline to a lesser extent. Exposure to light at 4 days, when the cotyledons emerge, results in a selectively greater destruction of enzymes of the glyoxylate cycle; chlorophyll synthesis and capacity for photosynthesis increase in parallel, and there is a striking increase in the activities of chloroplast enzymes and in those of the leaf peroxisomes, hydroxypyruvate reductase and glycolate oxidase. The reciprocal changes in enzymes of the glyoxysomes and of leaf peroxisomes can be temporally dissociated, since even after 10 days in darkness, when malate synthetase and isocitrate lyase have reached very low levels, hydroxypyruvate reductase and glycolate oxidase increase strikingly on exposure to light and the cotyledons become photosynthetic. Furthermore, the parallel development of enzymes of leaf peroxisomes and functional chloroplasts is not immutable, since hydroxypyruvate reductase and glycolate oxidase activity can be elicited in darkness following a 5-minute exposure to light at day 4 while chlorophyll does not develop under these conditions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breidenbach R. W., Kahn A., Beevers H. Characterization of glyoxysomes from castor bean endosperm. Plant Physiol. 1968 May;43(5):705–713. doi: 10.1104/pp.43.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierabend J., Beevers H. Developmental Studies on Microbodies in Wheat Leaves : II. Ontogeny of Particulate Enzyme Associations. Plant Physiol. 1972 Jan;49(1):33–39. doi: 10.1104/pp.49.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierabend J., Beevers H. Developmental studies on microbodies in wheat leaves : I. Conditions influencing enzyme development. Plant Physiol. 1972 Jan;49(1):28–32. doi: 10.1104/pp.49.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firenzuoli A. M., Vanni P., Ramponi G., Baccari V. Changes in Enzyme Levels During Germination of Seeds of Triticum durum. Plant Physiol. 1968 Feb;43(2):260–264. doi: 10.1104/pp.43.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt B. P., Beevers H. Developmental studies on glyoxysomes in Ricinus endosperm. J Cell Biol. 1970 Jan;44(1):94–102. doi: 10.1083/jcb.44.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURLBERT R. E., RITTENBERG S. C. Glucose metabolism of Euglena gracilis var. bacillaris; growth and enzymatic studes. J Protozool. 1962 May;9:170–182. doi: 10.1111/j.1550-7408.1962.tb02602.x. [DOI] [PubMed] [Google Scholar]
- Heber U., Pon N. G., Heber M. Localization of Carboxydismutase & Triosephosphate Dehydrogenases in Chloroplasts. Plant Physiol. 1963 May;38(3):355–360. doi: 10.1104/pp.38.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt A. J. Preparation & some properties of soluble succinic dehydrogenase from higher plants. Plant Physiol. 1961 Sep;36(5):552–557. doi: 10.1104/pp.36.5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P. C., Taylor J. M. Effects of organic acids on ion uptake and retention in barley roots. Plant Physiol. 1970 Oct;46(4):538–542. doi: 10.1104/pp.46.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A. O. Persistent photoreversibility of leaf development. Plant Physiol. 1969 Jun;44(6):897–902. doi: 10.1104/pp.44.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmak M., Tolbert N. E. Glycolic acid oxidase formation in greening leaves. Plant Physiol. 1962 Nov;37(6):729–734. doi: 10.1104/pp.37.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Development of Microbodies in Sunflower Cotyledons and Castor Bean Endosperm during Germination. Plant Physiol. 1971 Nov;48(5):566–574. doi: 10.1104/pp.48.5.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelease R. N., Becker W. M., Gruber P. J., Newcomb E. H. Microbodies (Glyoxysomes and Peroxisomes) in Cucumber Cotyledons: Correlative Biochemical and Ultrastructural Study in Light- and Dark-grown Seedlings. Plant Physiol. 1971 Oct;48(4):461–475. doi: 10.1104/pp.48.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Poucke M., Cerff R., Barthe F., Mohr H. Simultaneous induction of glycolate oxidase and glyoxylate reductase in white mustard seedlings by phytochrome. Naturwissenschaften. 1970 Mar;57(3):132–133. doi: 10.1007/BF00600062. [DOI] [PubMed] [Google Scholar]


