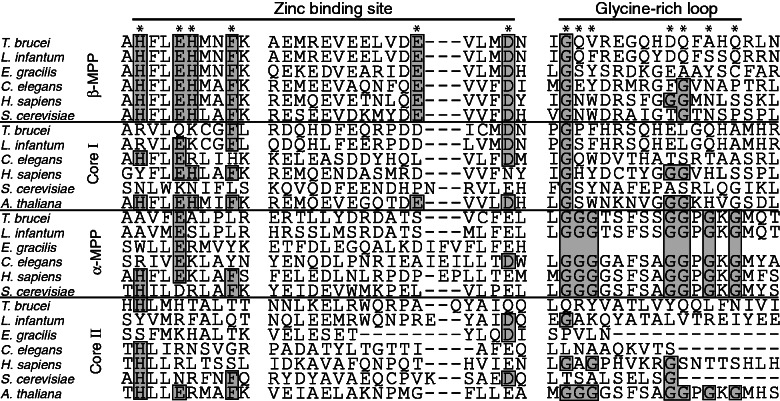
Fig. 2.—
Protein sequence alignment of MPP domains responsible for catalytic activity (zinc-binding site) and substrate recognition (glycine-rich loop). Positions of functionally important AA are marked by asterisks; conserved AA are in boxes. Accession numbers for protein sequences of α-MPP (Euglena gracilis, P43265; Trypanosoma brucei, XP_951618; Leishmania major, XP_001682967; Saccharomyces cerevisiae, NP_011889; Homo sapiens, NP_055975; Caenorhabditis elegans, NP_490888), β-MPP (E. gracilis, P43264; T. brucei, XP_803756; L. major, XP_003721587; S. cerevisiae, NP_013264; H. sapiens, NP_004270.2; C. elegans, NP_501576), cp1 (T. brucei, XP_844780; L. major, XP_001686023; S. cerevisiae, NP_009508; H. sapiens, NP_003356; Arabidopsis thaliana, NP_186858; C. elegans, NP_498202), and cp2 (E. gracilis, EC675176; T. brucei, XP_828477; L. major, XP_001681831; S. cerevisiae, NP_015517; H. sapiens, NP_003357; A. thaliana, NP_175610; C. elegans, NP_510011).