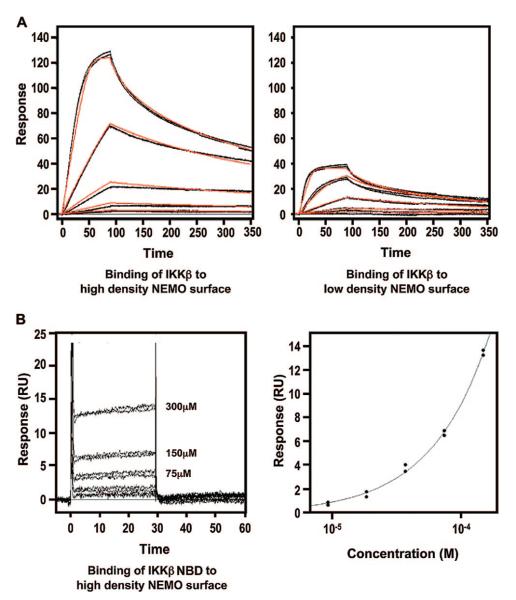
Figure 4.
Surface plasmon resonance (SPR) characterization of the IKKβ–NEMO interaction. (A) Binding of the IKKβ C-terminal domain (residues 680–756) to a NEMO (residues 38–196)-coupled sensor chip. The left and right panels show the binding of IKKβ (0.617–50.0 nM) to the high-density (left) and low-density (right) NEMO surfaces, respectively. Each of these data sets was fit to a 1:1 interaction model (red lines), and a KD of 3.4 nM was obtained. (B) Binding of the IKKβ NBD (residues 735–745) to a NEMO (residues 38–196)-coupled sensor chip. The left panel shows the binding of IKKβ (18.8–300.0 μM) to the high-density NEMO surface. The right panel shows the fitting of the responses at equilibrium against peptide concentration which yields a KD of 3.6 mM.