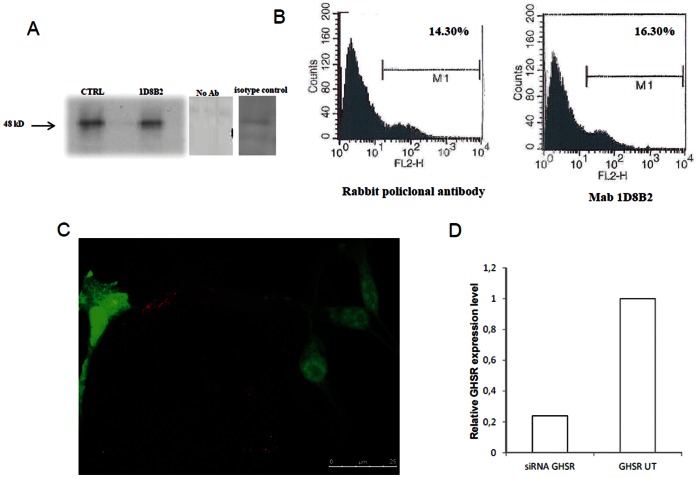
Figure 1. Production and characterization of Mab anti-GHS-R.
A. Immunoprecipitation of 22RV1 cell lysates with Mab 1D8B2 and commercial polyclonal antibody (CTRL). Lysates from cells were immunoprecipitated with Mab (8 µg) or polyclonal antibody, resolved and transferred to nitrocellulose membranes. The western blot analysis were performed with the polyclonal antibody when the monoclonal was used for immunoprecipitation and the other way around, both immunoprecipitation analysis clearly showed a 48 kD band corresponding to the predicted size of GHS-R. Sizes (kD) of molecular mass markers are indicated on the left. These experiments were performed independently at least twice with similar results. B. Binding analysis of Mab 1D8B2 to 22RV1 cells by flow cytometry. Purified monoclonal antibody (6 µg) were analyzed with a flow cytofluorimetric analysis to assess the binding of antibodies to the GHSR on the surface of the cells. The control consisted of an anti-GHSR purified polyclonal antibody. Experiments were performed four times with reproducible results C. Representative image of 22VR1 cells cotransfected with GHS-R siRNA and eGFP. eGFP (green) expressing cells do not show positivity for GHSR (red). D. Relative mRNA expression of GHS-R in transfected 22VR1 normalized on GAPDH. GHS-R expression is significantly lower in transfected cells(siRNA GHSR) than in non-transfected cells (GHSR UT).