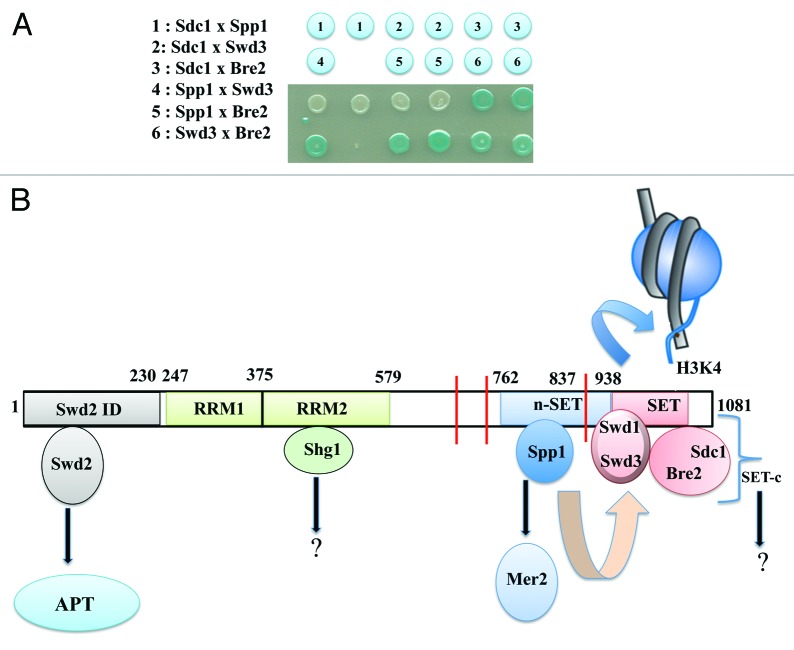
Figure 1. Organization of the Set1C and role of its subunits. (A) Protein/protein interactions within the indicated subunits of the Set1C were detected by the E. coli two-hybrid system.14 Interactions are revealed by the presence of β-galactosidase activity visualized on X-gal plates. (B) This scheme depicting the binding of the different Set1C subunits to Set1 is mainly based on8,11,15,17 (see text). The two RRM domains were positioned according to Trésaugues et al.18 Direct binding of Swd2, Shg1 and Spp1 to the indicated regions of Set1 has been established by Kim et al.17 The SET-c comprises Swd1-Swd3 and Bre2-Sdc1 modules that are likely to interact each other17 (see text and Figure 1A). In this model, Spp1 bound to the n-SET domain interacts with the SET-c via Swd3 to alter the enzyme catalytic pocket to facilitate H3K4me3. The SET-c could bind H3 through cooperative interactions between its subunits. Note that the PHD domain of Spp1 is not required for the H3K4 methylase activity of the Set1C. The N-terminal region of Set1 is thought to modulate the Set1C activity in a way that remains to be elucidated. Swd2 and Spp1 are known to interact independently of Set1 with APT and Mer2, respectively. We anticipate that Shg1 and the subunits of the SET-c may in turn establish alternative protein/protein interactions to regulate other processes than H3K4 methylation. Red bars indicate the position of putative PEST sequences. Numbers indicate amino acid residues.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.