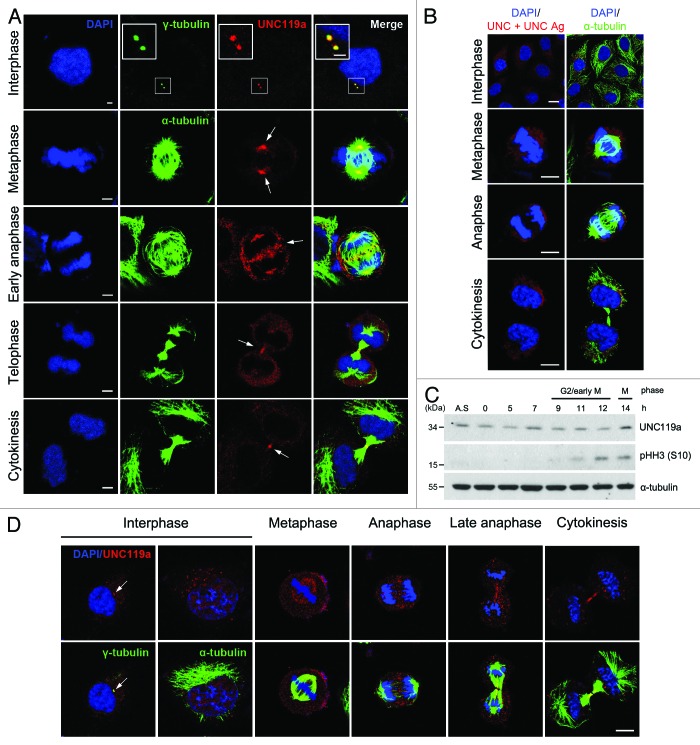
Figure 1. Cell cycle-dependent localization of UNC119a. Asynchronously growing HeLa cells were fixed in cold methanol (A and B) or paraformaldehyde (D). Fixed cells were double immunostained with UNC-Ab and monoclonal α-tubulin-Ab (or monoclonal γ-tubulin-Ab). The binding of the antibodies was visualized using Alexa 546-conjugated goat anti-rabbit IgG (red) and Alexa 488-conjugated goat anti-mouse IgG (green), respectively. The nuclei and chromosomes were stained with DAPI. (A) Cell cycle-dependent localization of UNC119a. White arrows show regions of highly concentrated UNC119a. Bars: 5 μm, in interphase; 10 μm, in other images. (B) UNC-Ab specifically recognizes endogenous UNC119a. Fixed HeLa cells were double immunostained with monoclonal α-tubulin-Ab and UNC-Ab pre-incubated with GST-UNC119a (UNC + UNC Ag). Pre-incubation of UNC-Ab with the antigen completely inhibited the binding of UNC-Ab to the endogenous protein. Bars, 20 μm. (C) The cellular content of UNC119 does not change during the cell cycle. HeLa cells were synchronized at G1/S using a double thymidine block. Synchronized cells were released into fresh medium, collected at the indicated times and lysed with RIPA buffer. Western blotting was performed with three antibodies: UNC-Ab for UNC119a; anti-phosphor-histone H3 (Ser 10)-Ab, pHH3 (S10), as a mitosis marker; and monoclonal α-tubulin-Ab as a loading control. A.S., asynchronous cell extracts. (D) Cell cycle-dependent localization of UNC119a in paraformaldehyde-fixed cells. Asynchronously growing HeLa cells were fixed with 4% paraformaldehyde and permeabilized in 0.2% Triton X-100 in PBS. Double immunostaining was performed as described above. White arrows indicate the centrosomes. Bar, 20 μm.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.