SUMMARY
All secretory anterior pituitary cells fire action potentials spontaneously and exhibit a high resting cation conductance, but the channels involved in the background permeability have not been identified. In cultured lactotrophs and immortalized GH3 cells, replacement of extracellular Na+ with large organic cations, but not blockade of voltage-gated Na+ influx, led to an instantaneous hyperpolarization of cell membranes that was associated with a cessation of spontaneous firing. When cells were clamped at -50 mV, which was close to the resting membrane potential in these cells, replacement of bath Na+ with organic cations resulted in an outward-like current, reflecting an inhibition of the inward holding membrane current and indicating loss of a background-depolarizing conductance. Quantitative RT-PCR analysis revealed the high expression of mRNA transcripts for TRPC1 and much lower expression of TRPC6 in both lactotrophs and GH3 cells. Very low expression of TRPC3, TRPC4, and TRPC5 mRNA transcripts were also present in pituitary but not GH3 cells. 2-APB and SKF-96365, relatively selective blockers of TRPC channels, inhibited electrical activity, Ca2+ influx and prolactin release in a concentration-dependent manner. Gd3+, a common Ca2+ channel blocker, and flufenamic acid, an inhibitor of non-selective cation channels, also inhibited electrical activity, Ca2+ influx and prolactin release. These results indicate that nonselective cation channels, presumably belonging to the TRPC family, contribute to the background depolarizing conductance and firing of action potentials with consequent contribution to Ca2+ influx and hormone release in lactotrophs and GH3 cells.
Keywords: TRPC channels, GH3 cells, calcium influx, action potentials, resting membrane potential
INTRODUCTION
The endocrine pituitary functions are carried out by corticotrophs secreting adrenocorticotrophic hormone, thyrotrophs secreting thyroid-stimulating hormone, somatotrophs secreting growth hormone, lactotrophs secreting prolactin (PRL), and gonadotrophs secreting luteinizing and follicle-stimulating hormones (Kelberman et al. 2009). The common characteristic of these cells, as well as of immortalized pituitary cells, is their spontaneous excitability. Firing of action potentials (APs) reflects the expression of numerous voltage-gated channels on the plasma membrane of these cells, including voltage-gated and tetrodotoxin (TTX)-sensitive Na+ (Nav) channels, voltage-gated Ca2+ (Cav) channels, voltage-gated K+ channels, calcium-regulated K+ channels, inwardly rectifying K+ channels, purinergic P2X channels, and GABAA channels (Kwiecien and Hammond 1998, Stojilkovic et al. 2010). Spontaneous firing of APs in all pituitary cell types leads to the activation of Cav channels and increased intracellular calcium ion concentration ([Ca2+]i), with the amplitude of transients determined by the pattern of firing (Stojilkovic at al. 2005). The T- and L-type Cav channels play a role in spike depolarization, while voltage-gated K+ channels and Ca2+-controlled K+ channels are responsible for plateau bursting and repolarization of cells (Van Goor et al. 2001a, Tsaneva-Atanasova et al. 2007).
The physiological significance of electrical activity in lactotrophs is well established. These cells in vitro release PRL in the absence of external stimuli, and such secretion is termed basal or spontaneous release (Freeman et al. 2000). High PRL release is also observed in animals bearing ectopic pituitary grafts (Maric et al. 1982). In both cases, spontaneous APs and the associated Ca2+ influx account for high steady-state PRL release and any maneuver leading to silencing of electrical activity also abolishes Ca2+ influx and basal PRL release (Van Goor et al. 2001a). In vivo, spontaneous electrical activity and PRL release are controlled by hypothalamic dopamine, acting through dopamine D2 receptors. Activation of these receptors leads to silencing of electrical activity through activation of inwardly rectifying K+ channels and inhibition of Cav channels (Missale et al. 1998). GH3 cells, an established immortalized cell line derived from a rat anterior pituitary tumor, maintain pituitary-specific behavior in culture by secreting PRL and growth hormone (Cronin et al. 1980). These cells are spontaneously electrically active with high amplitude APs and often serve as a convenient cell model in studying electrophysiological properties of lactotrophs and somatotrophs (Lo et al. 2001).
Channels contributing to the spike depolarization and repolarization in spontaneously firing cells have been identified (Stojilkovic et al. 2010). In contrast, very little is known about channels controlling resting membrane potential and initiation of firing of APs. Resting membrane potential in these cells is between -50 and -60 mV, positive to the equilibrium potassium potential, indicating the presence of depolarizing conductance. TTX-sensitive Nav channels are expressed in pituitary cells, but do not play important role in control of resting membrane potential and spike depolarization in lactotrophs in vitro (Van Goor et al. 2001b). A TTX-insensitive background Na+ (Nab) conductance was also identified in pituitary lactotrophs and GH3 immortalized cells as necessary for spontaneous depolarization and PRL release (Simasko 1994, Sankaranarayanan and Simasko 1996). However, the nature of these channels and the mechanism for their activation has not been identified.
Here we show that the abolition of Nab conductance by substituting extracellular Na+ with large organic cations leads to a rapid and reversible hyperpolarization of the plasma membrane and inhibition of firing of APs, Ca2+ influx and PRL secretion. Our results further indicate that this conductance takes place through non-selective cation channels, presumably the transient receptor potential - classic (TRPC) subfamily of these channels, which have been identified in pituitary cells using quantitative real-time PCR. TRPC channels conduct both Na+ and Ca2+, are voltage independent and their role in spontaneous and receptor controlled electrical activity and calcium signaling has been indicated in other excitable cells (Clapham et al. 2005).
METHODS
Animals and Cell Cultures
Experiments were performed on anterior pituitary cells from normal postpubertal female Sprague Dawley rats obtained from Taconic Farm (Germantown, MD). Euthanasia was performed by asphyxiation with CO2 and the anterior pituitary glands were removed after decapitation. Experiments were approved by the NICHD Animal Care and Use Committee. Anterior pituitary cells were mechanically dispersed after treatment with trypsin and cultured as mixed cells or enriched lactotrophs in medium 199 containing Earle’s salts, sodium bicarbonate, 10% heat-inactivated horse serum, penicillin (100 units/ml), and streptomycin (100 μg/ml). A two-stage Percoll discontinuous density gradient procedure was used to obtain enriched lactotrophs (Lussier et al. 1991). Further identification of lactotrophs in single cell studies was achieved by the addition of dopamine (Tocris Bioscience, Ellisville, MO) and TRH (Bachem, Torrance, CA). Immortalized GH3 pituitary cells were cultured in Ham’s F12K medium supplemented with 15% heat-inactivated horse serum, 2.5% fetal bovine serum, and gentamicin (100 μg/ml).
Electrophysiological Measurements
Pituitary cells were plated on poly-L-lysine coated cover slips (15 mm diameter) in densities of 100,000 primary cells per cover slip and 10,000 immortalized cells per cover slip and cultured for 1-3 days prior to recording. All recordings were performed at room temperature using Axopatch 200B amplifier (Molecular Devices, Union City, CA). The amphotericine perforated patch-clamp technique was used to record membrane potentials and whole cell currents. Cells were continuously perfused with an extracellular solution containing (in mM): 145 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES), and 10 glucose (pH 7.4). For Na+-free experiments, NaCl was replaced in 1:1 ratio by N-methyl-D-glucamine (NMDG), tetramethylammonium (TMA), or choline chloride (pH 7.4). Patch pipettes were pulled from borosilicate glass (World Precision Instruments, Sarasota, FL) and heat polished to a tip resistance of 5-7 MΩ. Pipette solution contained (in mM): 90 K-aspartate, 50 KCl, 3 MgCl2 and 10 HEPES (pH 7.2). Prior to measurement, amphotericine B was added to the pipette solution from a stock solution to obtain a final concentration of 200 μg/ml. Recordings started when series resistance dropped below 100 MΩ for current-clamp or below 40 MΩ for voltage-clamp recordings. Series resistance was compensated to more than 60%. Drugs dissolved to a final concentration in extracellular solutions were delivered to the recording chamber by a gravity-driven microperfusion system RSC-200 (Bio-Logic USA, Knoxville, TN).
Single cell intracellular calcium measurements
For measurements of [Ca2+]i, cells were incubated in Krebs Ringer buffer with 2 μM Fura-2 AM (Invitrogen, Carlsbad, CA) at room temperature for 60 min. For Na+-free experiments, NaCl was replaced by NMDG and pH was brought to 7.4 by adding HCl. Coverslips with cells were then washed with Krebs-Ringer buffer and mounted on the stage of an Observer-D1 microscope (Carl Zeiss, Oberkochen, Germany) attached to an ORCA-ER camera (Hamamatsu Photonics, Hamamatsu City, Japan) and a Lambda 10-B filter wheel (Sutter, Novato, CA) with 340 and 380 nm excitation filters (Carl Zeiss, Oberkochen, Germany). Hardware control and image analysis was performed using Metafluor software (Molecular Devices, Downingtown, PA). Cells were examined under an oil immersion objective during exposure to alternating 340- and 380-nm light beams, and the intensity of light emission at 520 nm was measured. The ratio of light intensities, F340/F380, which reflects changes in [Ca2+]i, was followed in several single cells simultaneously at the rate of one point per second.
Prolactin secretion
Hormone secretion was monitored using cell column perifusion experiments. Briefly, 1.5 × 107 cells were incubated with preswollen cytodex-1 beads in 60-mm petri dishes for 18 h. The beads were then transferred to 0.5-ml chambers and perifused with Hanks’ M199 containing 25 mM HEPES, 0.1% BSA, and penicillin (100 U/ml)/streptomycin (100 μg/ml) at 37 °C for 2.5 h at a flow rate of 0.8 ml/min to establish stable basal secretion. Fractions were collected in 1-min intervals and their PRL content was later determined using radioimmunoassay. Primary antibody and standard for PRL assay were purchased from the National Pituitary Agency and Dr. A. F. Parlow (Harbor-UCLA Medical Center, Torrance, CA). [125I]PRL was purchased from PerkinElmer Life Sciences (Boston, MA).
RT-PCR Analysis
Total RNA from the primary pituitary cells was extracted using the RNeasy Mini Kit. Subsequently, 1 μg of total RNA was treated with DNAse I and reverse transcribed with SuperScript III First Strand Synthesis SuperMix for qRT-PCR (all from Invitrogen, Carlsbad, CA). Quantitative RT-PCR was performed using pre-designed Taq-Man Gene Expression Assays (Applied Biosystems) with LightCycler® TaqMan® Master mix and LightCycler 2.0 Real-time PCR system (Roche Applied Science). Gene expression levels of the target genes were determined by the comparative 2ˆ(-delta delta C(T)) quantification method using GAPDH as a reference gene, where (delta delta C(T)) = (CT, target - CT, reference)sample - (CT, target - CT, reference)control. The Applied Biosystems pre-designed Taq-Man Gene Expression Assays were used: TRPC1-Rn00585625_m1; TRPC2-Rn00575304_m1; TRPC3-Rn00572928_m1; TRPC4-Rn00584835_m1; TRPC5-Rn00590142_m1; TRPC6-Rn00677564_m1; TRPC7-Rn01448763_m1; GAPDH-Rn01462662_g1.
RESULTS
Dependence of electrical activity and PRL release on the background Na+ conductance
To determine the role of Nab conductance in resting membrane potential and spontaneous electrical activity of pituitary cells, we first examined the effect of Na+ removal from extracellular solution. We used lactotrophs and GH3 cells that tend to fire APs spontaneously (Fig. 1A and C). However, the fraction of active cells is lower in lactotrophs than in GH3 cells: about 60% and 90%, respectively. TTX, a Nav channel blocker, was ineffective in abolishing spontaneous electrical activity (Fig. 1C) in concentrations up to 10 μM. In contrast, replacement of extracellular Na+ with NMDG led to an instantaneous hyperpolarization of cell membranes both in lactotrophs (-15.1 ± 2.2 mV; n=6) and GH3 cells (-28.3 ± 7.2 mV; n=10). The hyperpolarization was associated with a cessation of spontaneous firing of APs (Fig. 1A). Once the cells were returned to Na+-containing physiological buffer, the spontaneous electrical activity resumed. We also frequently observed an increase in the firing frequency during the initial period of the recovery phase (Fig. 1A), which could indicate that the level of [Ca2+]i determines the frequency of spontaneous firing of APs. These experiments show that basal sodium conductance controls resting membrane potential and electrical activity in pituitary cells.
Fig. 1.
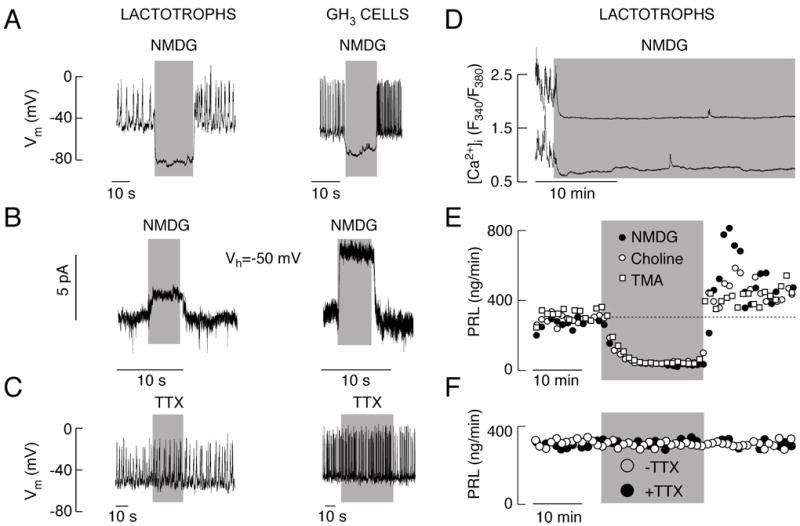
Spontaneous electrical activity, calcium influx and basal prolactin (PRL) release of pituitary cells depend on a background Na+ conductance. A and B, Effects of complete replacement of extracellular Na+ with NMDG on electrical activity (A) and whole cell current (B) in lactotrophs (left panels) and GH3 cells (right panels). Note the increased firing frequency after return to normal Na+-containing bath buffer. Vh, holding potential. C, The lack of effects of 1 μM TTX, a Nav channel blocker, on membrane potential (Vm) and spontaneous firing of APs in lactotrophs (left panel) and GH3 cells (right panel). D, Effect of complete replacement of bath Na+ with NMDG on spontaneous calcium transients in lactotrophs. In this and following figures, data shown are representative of at least 5 recordings. Similar effects of replacement of bath Na+ with NMDG were observed in other pituitary cell types. E, Effect of complete replacement of bath Na+ with NMDG, TMA and choline chloride on basal PRL release in perifused pituitary cells. In parallel to electrical activity (Fig. 1A, left), notice the transient increase in PRL release after return of Na+ containing buffer. F, The lack of effect of TTX on basal PRL release. Gray areas indicate duration of treatments.
In further experiments we asked whether calcium influx and hormone secretion are regulated by Nab conductance. We clamped cells at -50 mV, a physiologically relevant voltage because the resting potentials in these cells were close to that value (Fig. 1A and 1C). In both cell types, TTX had no effect on holding current (data not shown), while complete replacement of extracellular Na+ with NMDG caused a shift in the baseline, with the appearance of an outward current (Fig. 1B). A decrease in the noise of recording during NMDG application suggested that the shift in the baseline was due to a closure of cation channels and loss of a background depolarizing conductance. The amplitude of this outward-like current was 2.1±0.3 pA (n=7) in lactotrophs and 9.9±1.7 pA (n=13) in GH3 cells. It was not abolished by the blockade of L-type Cav channels with nimodipine or by the inhibition of hyperpolarization-activated HCN channels with 1 mM Cs+ (data not shown). The same qualitative effects on membrane voltage and current were observed by substituting bath Na+ with other large organic cations, including TMA and choline (data not shown).
Removal of bath Na+ also led to cessation of calcium transients and rapid decrease in [Ca2+]i basal levels (Fig. 1D). Figures 1E and 1F show that basal PRL release from primary rat pituitary cells is high, indicating that spontaneous [Ca2+]i transients are sufficient to trigger hormone secretion. A transient replacement of Na+ with large organic cations abolished such elevated basal PRL release by perifused pituitary cells (Fig. 1E), while TTX had no effect on hormone secretion (Fig. 1F). Thus, spontaneous firing of APs and accompanied [Ca2+]i transients in lactotrophs and GH3 cells depends on TTX-insensitive Nab conductance, and such electrical activity is critical for basal PRL release.
Dependence of electrical activity and PRL release on Ca2+ conductance
To resolve the identity of the channels contributing to Nab conductance-dependent Ca2+ influx and PRL release, we first used Gd3+, an inhibitor of various Ca2+-conducting channels, including Cav and TRPC channels (Clapham et al. 2005, Biagi and Enyeart 1990, Lacampagne et al. 1994). Electrophysiological experiments in single GH3 cells and lactotrophs showed that Gd3+ in 2 μM concentration not only abolished firing of APs, but also hyperpolarized the cell membrane (Fig. 2A). However, this hyperpolarization was smaller than the one caused by removal of bath Na+ (Fig. 1A). In single lactotrophs, addition of GdCl3 abolished spontaneous calcium transients (Fig. 2B) similarly as replacement of Na+ with NMDG (Fig. 1D). In perifused pituitary cells, Gd3+ inhibited basal PRL release in a concentration-dependent manner, with an IC50 value of about 2 μM (Fig. 2C). This raises the possibility that Nab channels conduct Ca2+ in addition to Na+, i.e. that some of the non-selective cation channels account for or contribute to Nab conductance. Consistent with this hypothesis, flufenamic acid (FFA), a generic blocker of non-selective cation channels (Egorov et al. 2002, Ghamari-Langroudi and Bourque 2002), also abolished spontaneous firing of APs in GH3 cells, and hyperpolarized the cell membrane by 5-10 mV (Fig. 3A). Furthermore, FFA inhibited spontaneous [Ca2+]i transients in TRH-indentified lactotrophs (Fig. 3B) and basal PRL release in perifused pituitary cells (Fig. 3C). These physiological and pharmacological responses indicate that non-selective cation channels are involved in the background depolarization of pituitary lactotrophs and GH3 cells.
Fig. 2.
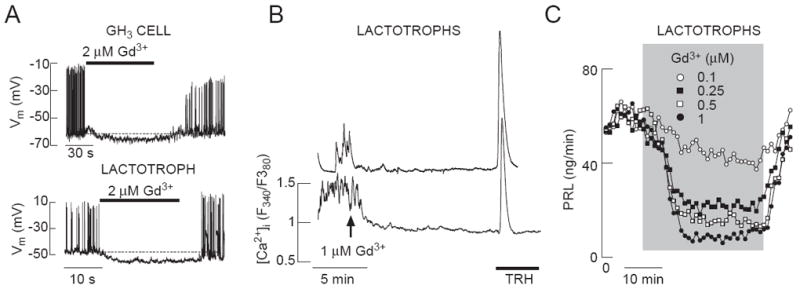
Effects of Gd3+, a non-specific blocker of calcium-conducting channels, on electrical activity in GH3 cells and lactotrophs (A), calcium influx in lactotrophs (B) and PRL release in perifused pituitary cells (C) Notice the Gd3+-induced hyperpolarization of the plasma membrane in A.
Fig. 3.
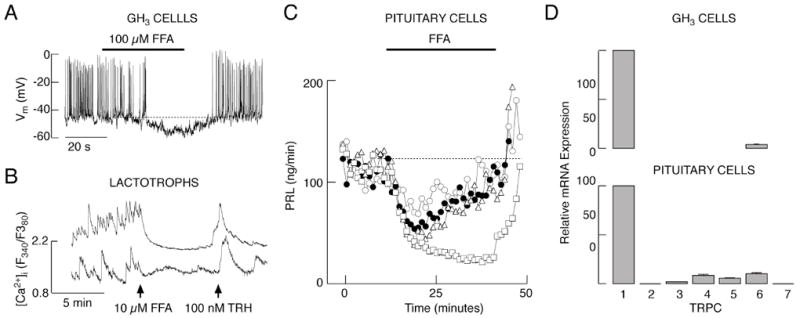
Effects of flufenamic acid (FFA), a blocker of various non-selective cation channels, on electrical activity in GH3 cells (A), calcium influx in lactotrophs (B) and PRL release in perifused pituitary cells (from top to bottom 25, 50, 75 and 100 μM) (C). D, Quantitative RT-PCR analysis of TRPC-mRNA expression in GH3 cells (top). Data shown are mean ± SEM values of six experiments, using TRPC1-mRNA expression as 100%. Results for pituitary cells (bottom panel) are derived from Tomic et al. 2011. Notice the FFA-induced hyperpolarization of the plasma membrane in A.
Expression and contribution of TRPC channels to Nab conductance
In further work, we collected more evidence on expression and active involvement of TRPC subfamily of these channels in GH3 cells. These cells express TRPC1 and TRPC6 mRNA transcripts (Fig. 3D, top), whereas pituitary cells also express mRNA transcripts for TRPC2, TRPC3, TRPC4, TRPC5 and TRPC7 (Fig. 3D, bottom). SKF96365, a widely used and relatively specific inhibitor of TRPC channels (Clapham et al. 2005), inhibited the electrical activity in GH3 cells (data not shown). In lactotrophs, 10 μM SKF96365 inhibited [Ca2+]i transients (Fig. 4A), in some cells after a transient increase in [Ca2+]i (data not shown). Consistent with these results, application of SKF96365 decreased PRL secretion from perifused pituitary cells in a concentration-dependent manner (Fig. 4B). 2-APB, another blocker of TRPC channels (Clapham et al. 2005), had similar effects on AP firing in GH3 cells (not shown) and basal PRL secretion in perifused pituitary cells (Fig. 4C). PRL secretion was inhibited in a concentration dependent manner with an IC50 value of 38 μM. These results suggest that TRPC channels contribute to Nab conductance in lactotrophs and GH3 cells, and that their contribution is physiologically relevant.
Fig. 4.
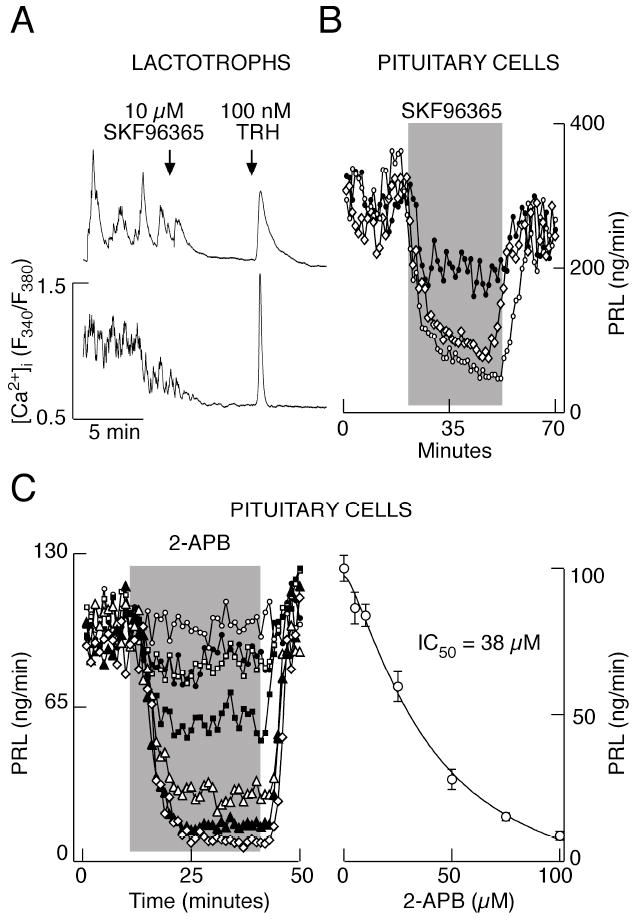
Effects of specific blockers of TRPC channels on calcium influx and PRL release. A, Inhibition of spontaneous calcium influx by SKF96365 in lactotrophs. B, Dose-dependent effect of SKF96365 on basal PRL release. From top to bottom: 10, 25, 50 μM SKF96365. C, Dose-dependent effects of 2-APB on basal PRL release. Left, representative records in response to 0, 5, 10, 25, 50, 75 and 100 μM 2-APB (from top to bottom). Right, concentration-dependence study revealed the IC50 value of 38 μM. Mean ± SEM values of four experiments are shown.
DISCUSSION
Lactotrophs and GH3 cells fire APs spontaneously and such electrical activity drives transients in [Ca2+]i (Van Goor et al. 2001a, Mollard et al. 1996, Schlegel et al. 1987) of sufficient amplitude to trigger PRL release (Zorec 1996). The role of bath Ca2+ and Cav channels in both spontaneous firing of APs and basal PRL release is well established. Basal PRL release is abolished in cells with blocked Cav channels, which are responsible for spike depolarization and the accompanied Ca2+ influx (Van Goor et al. 2001a). This is a unique characteristic of lactotrophs and somatotrophs; in other anterior pituitary cell types, spontaneous electrical activity does not cause significant hormone secretion (Stojilkovic et al. 1988).
In this study, we examined the role of bath Na+ in electrical activity, Ca2+ signaling and PRL release. We showed that replacement of bath Na+ with large organic cations resulted in the membrane hyperpolarization and consequent abolition of spontaneous AP firing in pituitary lactotrophs and GH3 cells. Experiments with voltage-clamped cells at resting membrane potential further revealed that replacement of bath Na+ with organic cations caused a loss of basal depolarizing conductance. The Na+ conductance is TTX-insensitive, it determines the resting membrane potential, and is necessary for the spontaneous firing of Ca2+-dependent APs as well as the associated Ca2+ transients and PRL release. Others published similar observations in GH3 cells (Simasko 1994) and lactotrophs (Sankaranarayanan and Simasko 1996), and termed this conductance Nab conductance. Its presence is not unique to PRL-secreting cells, but was also observed in other endocrine pituitary cells, including somatotrophs and gonadotrophs (Kucka et al. 2010). We have reported recently that Nab conductance plays an important role in multidrug-resistance protein-mediated cyclic nucleotide efflux in anterior pituitary cells (Kucka et al. 2010). However, the nature of the channels that enable this conductance and the mechanism for its activation has not been clarified.
Recently, we have also reported that Nab conductance could be mediated by TRPC channels, the largest family of cation-conducting channels (Tomic et al. 2011). In general, their activation leads to Na+ and Ca2+ influx and consequent depolarization of the plasma membrane (Clapham et al. 2005). Consistent with the role of Nab current in the control of resting membrane potential in normal and immortalized pituitary cells, some TRPC channels are constitutively active (Trebak et al. 2003, Nichols et al. 2007). Furthermore, TRPC4 was suggested as a molecular candidate for the nonselective cation channel responsible for the pacemaker activity in interstitial cells of Cajal (Kim et al. 2006). TRPC cation channels mediating persistent muscarinic currents also contribute significantly to the firing and mnemonic properties of projection neurons in the entorhinal cortex (Zhang et al. 2011). Leptin also depolarizes the plasma membrane via activation of TRPC channels in guinea pig kisspeptin neurons and proopiomelanocortin neurons (Qiu et al. 2010, Qui et al. 2011). These channels also contribute to agonist-induced depolarization of hypothalamic gonadotropin-releasing neurons (Zhang et al. 2008).
With such a tissue-wide distribution and involvement in electrical activity, here we give preliminary evidence for the further role of TRPC channels in Nab conductance, Ca2+ signaling and hormone secretion of pituitary lactotrophs and GH3 lacto-somatotrophs. The mRNA transcripts for TRPC1 channels are highly expressed in normal and immortalized pituitary cells, whereas the expression of other subunits of this family of channels is below 5% of TRPC1-mRNAs. The blockers of TRPC channels, SKF96365 and 2-APB, and the nonselective blockers of cation channels including TRPC channels, FFA and Gd3+, hyperpolarize the plasma membrane and inhibit spontaneous firing of APs, accompanied calcium influx and basal PRL release. Therefore, a TTX-resistant background-depolarizing Na+ channels, presumably belonging to the TRPC family, are critical for firing of APs, accompanied Ca2+ influx, and PRL release in lactotrophs and GH3 cells.
Acknowledgments
The authors were supported by the Intramural Research Program of the National Institute of Child Health and Human Development, the Grant Agency of the Czech Republic (305/07/0681) and the Project Excellence (P304/12/G069).
Footnotes
Authors have nothing to declare
References
- BIAGI BA, ENYEART JJ. Gadolinium blocks low- and high-threshold calcium currents in pituitary cells. Am J Physiol. 1990;259:C515–C520. doi: 10.1152/ajpcell.1990.259.3.C515. [DOI] [PubMed] [Google Scholar]
- CLAPHAM DE, JULIUS D, MONTELL C, SCHULTZ G. International Union of Pharmacology XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev. 2005;57:427–450. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- CRONIN MJ, FAURE N, MARTIAL JA, WEINER RI. Absence of high affinity dopamine receptors in GH3 Cells: a prolactin-secreting clone resistant to the inhibitory action of dopamine. Endocrinology. 1980;106:718–723. doi: 10.1210/endo-106-3-718. [DOI] [PubMed] [Google Scholar]
- EGOROV AV, HAMAM BN, FRANSEN E, HASSELMO ME, ALONSO AA. Graded persistent activity in entorhinal cortex neurons. Nature. 2002;420:173–178. doi: 10.1038/nature01171. [DOI] [PubMed] [Google Scholar]
- FREEMAN ME, KANYICSKA B, LERANT A, NAGY G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- GHAMARI-LANGROUDI M, BOURQUE CW. Caesium blocks depolarizing after-potentials and phasic firing in rat supraoptic neurones. J Physiol. 2002;545:537–542. doi: 10.1111/j.1469-7793.1998.165bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELBERMAN D, RIZZOTI K, LOVELL-BADGE R, ROBINSON IC, DATTANI MT. Genetic regulation of pituitary gland development in human and mouse. Endocr Rev. 2009;30:790–829. doi: 10.1210/er.2009-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM BJ, SO I, KIM KW. The relationship of TRP channels to the pacemaker activity of interstitial cells of Cajal in the gastrointestinal tract. J Smooth Muscle Res. 2006;42:1–7. doi: 10.1540/jsmr.42.1. [DOI] [PubMed] [Google Scholar]
- KUCKA M, KRETSCHMANNOVA K, MURANO T, WU CP, ZEMKOVA H, AMBUDKAR SV, STOJILKOVIC SS. Dependence of multidrug resistance protein-mediated cyclic nucleotide efflux on the background sodium conductance. Mol Pharmacol. 2010;77:270–279. doi: 10.1124/mol.109.059386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KWIECIEN R, HAMMOND C. Differential management of Ca2+ oscillations by anterior pituitary cells: A comparative overview. Neuroendocrinology. 1998;68:135–151. doi: 10.1159/000054360. [DOI] [PubMed] [Google Scholar]
- LACAMPAGNE A, GANNIER F, ARGIBAY J, GARNIER D, LE GUENNEC JY. The stretch-activated ion channel blocker gadolinium also blocks L-type calcium channels in isolated ventricular myocytes of the guinea-pig. Biochim Biophys Acta. 1994;1191:205–208. doi: 10.1016/0005-2736(94)90250-x. [DOI] [PubMed] [Google Scholar]
- LO YK, WU SN, LEE CT, LI HF, CHIANG HT. Characterization of action potential waveform-evoked L-type calcium currents in pituitary GH3 cells. Pflugers Arch. 2001;442:547–557. doi: 10.1007/s004240100576. [DOI] [PubMed] [Google Scholar]
- LUSSIER BT, FRENCH MB, MOOR BC, KRAICER J. Free Intracellular Ca2+ Concentration ([Ca2+]i) and Growth Hormone Release from Purified Rat Somatotrophs. I. GH-Releasing Factor-Induced Ca2+ Influx Raises [Ca2+]i. Endocrinology. 1991;128:570–582. doi: 10.1210/endo-128-1-570. [DOI] [PubMed] [Google Scholar]
- MARIC D, SIMONOVIC I, KOVACEVIC R, KRSMANOVIC L, STOJILKOVIC SS, ANDJUS RK. Effects of short-term and long-term hypeprolactinema on the developmental pattern of androgen and LH levels in the immature male rats. J Endocrinol Invest. 1982;5:235–241. doi: 10.1007/BF03348329. [DOI] [PubMed] [Google Scholar]
- MISSALE C, NASH SR, ROBINSON SW, JABER M, CARON MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- MOLLARD P, SCHLEGEL W. Why are endocrine pituitary cells excitable? Trends Endocrinol Metab. 1996;7:361–365. doi: 10.1016/s1043-2760(96)00186-5. [DOI] [PubMed] [Google Scholar]
- NICHOLS RA, DENGLER AF, NAKAGAWA EM, BASHKIN M, PAUL BT, WU J, KHAN GM. A constitutive, transient receptor potential-like Ca2+ influx pathway in presynaptic nerve endings independent of voltage-gated Ca2+ channels and Na+/Ca2+ exchange. J Biol Chem. 2007;282:36102–36111. doi: 10.1074/jbc.M706002200. [DOI] [PubMed] [Google Scholar]
- QIU J, FANG Y, BOSCH MA, RONNEKLEIV OK, KELLY MJ. Guinea Pig Kisspeptin Neurons Are Depolarized by Leptin via Activation of TRPC Channels. Endocrinology. 2011;152:1503–1514. doi: 10.1210/en.2010-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QIU J, FANG Y, RONNEKLEIV OK, KELLY MJ. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci. 2010;30:1560–1565. doi: 10.1523/JNEUROSCI.4816-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANKARANARAYANAN S, SIMASKO SM. A role for a background sodium current in spontaneous action potentials and secretion from rat lactotrophs. Am J Physiol. 1996;271:C1927–C1934. doi: 10.1152/ajpcell.1996.271.6.C1927. [DOI] [PubMed] [Google Scholar]
- SCHLEGEL W, WINIGER BP, MOLLARD P, VACHER P, WUARIN F, ZAHND GR, WOLLHEIM CB, DUFY B. Oscillations of cytosolic Ca2+ in pituitary cells due to action potentials. Nature. 1987;329:719–721. doi: 10.1038/329719a0. [DOI] [PubMed] [Google Scholar]
- SIMASKO SM. A background sodium conductance is necessary for spontaneous depolarizations in rat pituitary cell line GH3. Am J Physiol. 1994;266:C709–C719. doi: 10.1152/ajpcell.1994.266.3.C709. [DOI] [PubMed] [Google Scholar]
- STOJILKOVIC SS, IZUMI S, CATT KJ. Participation of voltage-sensitive calcium channels in pituitary hormone release. J Biol Chem. 1988;263:13054–13061. [PubMed] [Google Scholar]
- STOJILKOVIC SS, TABAK J, BERTRAM R. Ion channels and signaling in the pituitary gland. Endocr Rev. 2010;31:845–915. doi: 10.1210/er.2010-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOJILKOVIC SS, ZEMKOVA H, VAN GOOR F. Biophysical basis of pituitary cell type-specific Ca2+ signaling-secretion coupling. Trends Endocrinol Metab. 2005;16:152–159. doi: 10.1016/j.tem.2005.03.003. [DOI] [PubMed] [Google Scholar]
- TOMIC M, KUCKA M, KRETSCHMANNOVA K, LI S, NESTEROVA M, STRATAKIS CA, STOJILKOVIC SS. Role of nonselective cation channels in spontaneous and protein kinase A-stimulated calcium signaling in pituitary cells. Am J Physiol Endocrinol Metab. 2011;301:E370–E379. doi: 10.1152/ajpendo.00130.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREBAK M, VAZQUEZ G, BIRD GS, PUTNEY JW., Jr The TRPC3/6/7 subfamily of cation channels. Cell Calcium. 2003;33:451–461. doi: 10.1016/s0143-4160(03)00056-3. [DOI] [PubMed] [Google Scholar]
- TSANEVA-ATANASOVA K, SHERMAN A, VAN GOOR F, STOJILKOVIC SS. Mechanism of spontaneous and receptor-controlled electrical activity in pituitary somatotrophs: experiments and theory. J Neurophysiol. 2007;98:131–144. doi: 10.1152/jn.00872.2006. [DOI] [PubMed] [Google Scholar]
- VAN GOOR F, ZIVADINOVIC D, MARTINEZ-FUENTES AJ, STOJILKOVIC SS. Dependence of pituitary hormone secretion on the pattern of spontaneous voltage-gated calcium influx. Cell type-specific action potential secretion coupling. J Biol Chem. 2001a;276:33840–33846. doi: 10.1074/jbc.M105386200. [DOI] [PubMed] [Google Scholar]
- VAN GOOR F, ZIVADINOVIC D, STOJILKOVIC SS. Differential expression of ionic channels in rat anterior pituitary cells. Mol Endocrinol. 2001b;15:1222–1236. doi: 10.1210/mend.15.7.0668. [DOI] [PubMed] [Google Scholar]
- ZHANG C, ROEPKE TA, KELLY MJ, RONNEKLEIV OK. Kisspeptin depolarizes gonadotropin releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci. 2008;28:4423–4434. doi: 10.1523/JNEUROSCI.5352-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG Z, REBOREDA A, ALONSO A, BARKER PA, SEGUELA P. TRPC channels underlie cholinergic plateau potentials and persistent activity in entorhinal cortex. Hippocampus. 2011;21:386–397. doi: 10.1002/hipo.20755. [DOI] [PubMed] [Google Scholar]
- ZOREC R. Calcium signaling and secretion in pituitary cells. Trends Endocrinol Metab. 1996;7:384–388. doi: 10.1016/s1043-2760(96)00169-5. [DOI] [PubMed] [Google Scholar]


