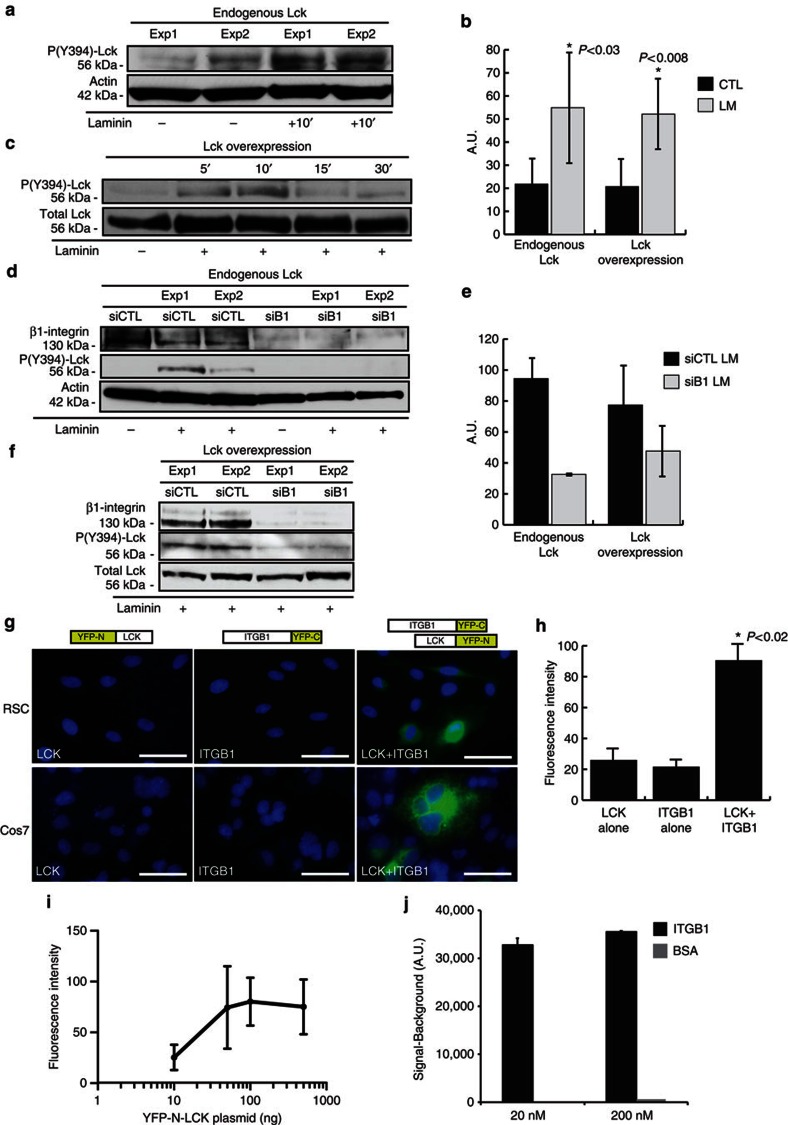
Figure 1. Laminin-induced interaction of β1-integrin with Lck mediates Lck phosphorylation in SCs.
(a,b) Active site (Y394) phosphorylation of endogenous Lck is induced after 10-min incubation with laminin (15 μg ml−1) in serum-starved SCs. Panel depicts representative western blot from two independent experiments. (b) Graph shows densitometric quantification of western blots for Lck phosphorylation from endogenous Lck (P<0.03, n=4) (a) and overexpressed Lck (P<0.008, n=5) (c), error bars represent ±s.d. (c) SCs overexpressing Lck were treated with 15 μg ml−1 laminin, and lysates were analysed for Lck phosphorylation. Lck is transiently activated (5–10 min) in response to laminin treatment. Quantification of Lck phosphorylation is shown in b. (d–f) Phosphorylation of endogenous Lck (d) or overexpressed Lck (f) was reduced following downregulation of β1-integrin with a specific siRNA but not with a control non-targeting siRNA, and quantification of western blots is shown (e, n=2). (g) In vivo interaction between Lck and β1-integrin is identified by BiFC in SCs and Cos7 cells. BiFC constructs were generated by fusing Lck to YFP-N (a.a. 1–158) and β1-integrin to YFP-C (a.a. 159–235). Coexpression in both SCs and Cos7 cells showed YFP fluorescence at the cell membrane. Expression of either construct alone did not produce YFP fluorescence. Scale bars represent 50 μm. (h) Average fluorescence intensities of YFP constructs. Intensity values correspond to 3-s exposure with background removed. Expression of Lck-YFP-N or ITGB1-YFP-C alone represents cell autofluorescence. Cells below this intensity level were not analysed in the LCK+ITGB1 experiments. Average fluorescence intensity was significantly increased during coexpression of Lck-YFP-N and ITGB1-YFP-C in Cos7 cells (P<0.03 vs Lck alone and ITGB1 alone, n=2 independent experiments, error bars represent ±s.d.). (i) Cell fluorescence intensity correlates to increasing YFP-N-LCK expression in Cos7 cells (n=3 independent experiments, y axis=log10, error bars represent ±s.d). (j) Active Lck binds directly to β1-integrin in a chemiluminescence binding assay using recombinant human proteins (Meso Scale Discovery). Representative signal measurements are shown for active Lck bound to β1-integrin, with saturating concentrations of active Lck and β1-integrin of 20 nM and 200 nM (n=duplicate wells, error bars represent ±s.d). Non-specific binding (BSA) showed a negligible signal. Statistical significance was determined by two-tailed Student’s t-test.