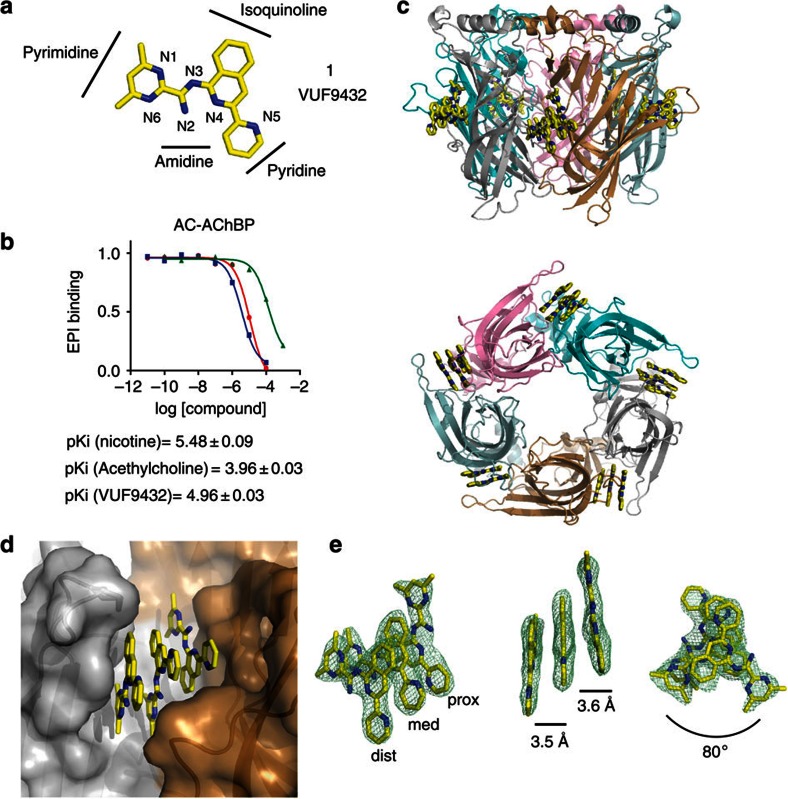
Figure 1. VUF9432 binds as a triple stack to Ac-AChBP.
(a) Chemical structure of VUF9432 (carbon atoms in yellow, nitrogen atoms in blue). (b) Displacement of radio-labelled epibatidine (EPI) by VUF9432 (red curves), nicotine (blue curves) and acetylcholine (green curves) on Ac-AChBP (purified proteins). Data are the mean±s.e.m. of three experiments and are reported below the panel. (c) Side and bottom side view of Ac-AChBP-VUF9432 complex structure, showing VUF9432 molecules (yellow sticks) in the five binding sites. (d) Protomer–protomer interfaces (surface representation) of Ac-AChBP-VUF9432 complex. Principal side is depicted in silver and complementary side in sand colours. Ligand-binding site are shown in transparency. (e) Electron density map displaying VUF9432 molecules in the ligand-binding site formed by subunit A and B (experimental density contoured at 1 σ), different orientation of the stacking molecules are shown together with the nomenclature used to distinguish the three molecules in the text. Intermolecular distances and angles are depicted as lines between the planes. dist, distal; med, medial; prox, proximal.