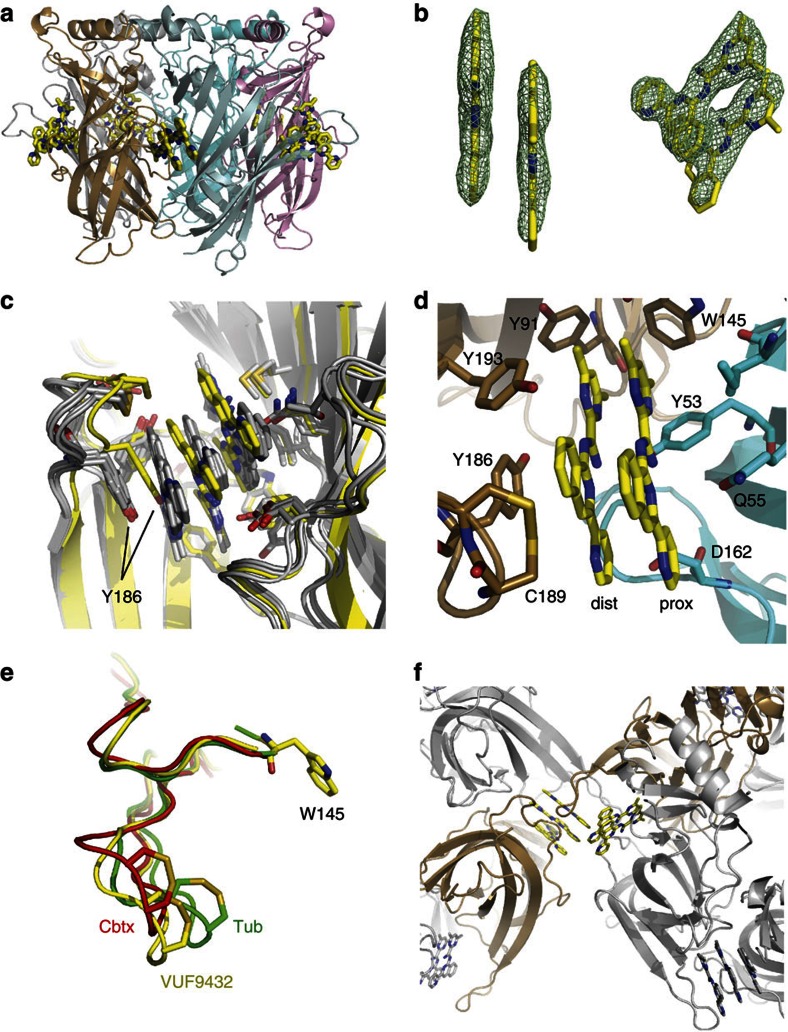
Figure 3. AChBP can accommodate a double stack of VUF9432.
(a) Side view of Ac-AChBP-VUF9432 complex structure, showing two VUF9432 molecules (yellow sticks) in the binding site formed by subunit B (sand) and C (cyan). (b) Electron density map displaying VUF9432 molecules in the ligand-binding site formed by subunit B and C (experimental density contoured at 1 σ). (c) Superposition of the four ligand binds site binding triple stacks of VUF9432 (grey cartoon and sticks) and the one binding the double stack of molecule (yellow cartoon and sticks), showing the movement of the Y186 to accommodate the third molecule of the stack. (d) Ball-and-stick representation showing AChBP residues on principal and complementary subunits interacting with the double stack of VUF9432 (colours as in a). (e) Comparison of C-loop opening in AChBP-tubocurarine (Tub, green), AChBP-α-cobratoxin (Cbtx, red), AChBP-VUF9432 (the shown C-loop is the one of the ligand-binding site containing two copies of the stack, yellow) complex. Position assumed by the C-loop after VUF9432 binding resembles that in cobratoxin. (f) Interaction of an AChBP-VUF9432 molecule with the symmetry-related molecule that form intermolecular contacts. The open C-loop of subunit B contacts the F-loop of a symmetry-related molecules. The bound VUF9432 molecules are shown in ball and sticks.