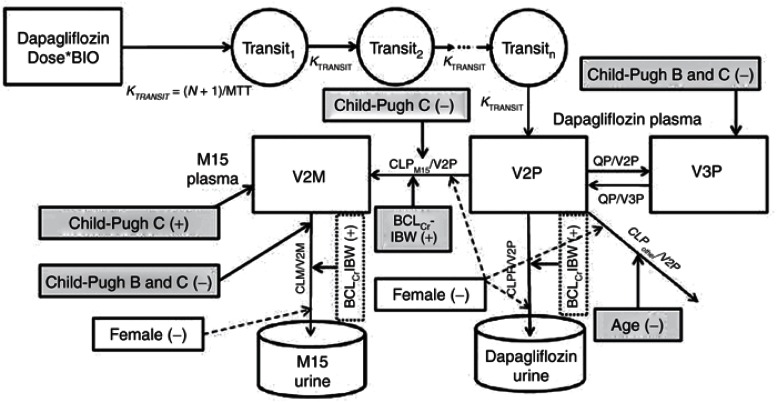
Figure 1.
The structural and covariate model to describe the renal and nonrenal elimination of dapagliflozin and D3OG in healthy subjects, T2DM subjects with normal or impaired renal function, and patients with hepatic impairment. BCLCrIBW, baseline creatinine clearance calculated using ideal body weight; CLM, renal clearance of D3OG; BIO, bioavailability; CLPM15, metabolic clearance of dapagliflozin to D3OG; CLPother, metabolic clearance of dapagliflozin to unmeasured metabolites; CLPrenal, renal clearance of unchanged dapagliflozin to urine; MTT, mean transit time; N, number of transit compartments; QP, intercompartmental clearance of dapagliflozin; T2DM, type 2 diabetes mellitus; V2P, central volume of distribution of dapagliflozin; V2M, central volume of distribution of D3OG; V3P, peripheral volume of distribution of dapagliflozin. Dashed lines, a priori scaling; shaded areas, covariates selected during stepwise covariate model building; unshaded areas, added based on previous modeling experience. Covariates connected to compartments affect the relevant volume, those connected to pathways affect the relevant clearance.