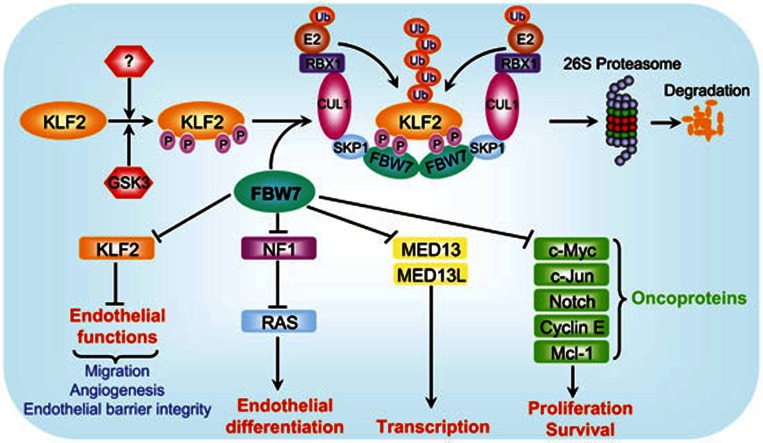
Figure 1.
A model for SCFFBW7-mediated KLF2 degradation to regulate endothelial functions. Tumor suppressor FBW7 is a substrate-recognizing subunit of SCF E3 ubiquitin ligase and promotes degradation of a) oncoproteins to regulate proliferation and survival; b) NF1 to modulate endothelial differentiation; and c) MED13/MED13L to regulate transcription. A new study reported in this issue of Cell Research revealed that KLF2, a transcription factor that negatively regulates endothelial functions, is also a substrate of SCFFBW7 E3 ligase. Upon phosphorylation by GSK3 and likely other kinases under an undefined physiological trigger(s), KLF2 is recognized by FBW7 via two CPD motifs. Dimerization of SCFFBW7 E3 ligase promotes KLF2 ubiquitylation and subsequent degradation by the 26S proteasome. Thus, by targeted degradation of KLF2, SCFFBW7 activates endothelial functions, including migration, angiogenesis and the maintenance of endothelial barrier integrity.