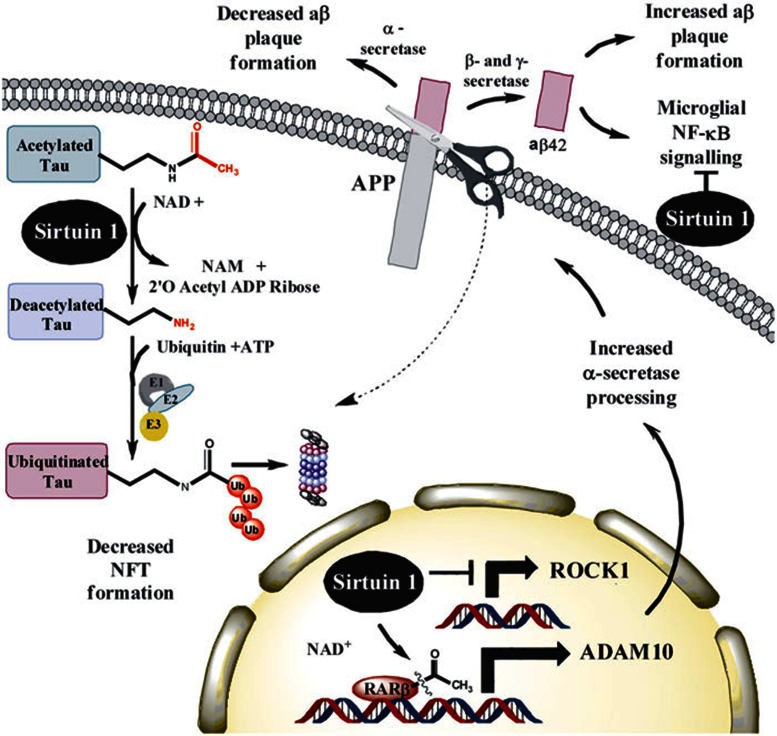
Figure 2.
Mechanisms of activity for sirtuins in Alzheimer's disease. In vitro and neuronal culture data show that SIRT1 can directly deacetylate tau protein at multiple residues62,63,64. The mechanism proposed by these investigators is that removal of acetyl groups may expose lysine residues to ubiquitin ligases so that tau protein is marked for proteasomal degradation62,63. This process decreases accumulation of hyperphosphorylated PHF tau, cognitive defects and early mortality in the P301L tau mouse model62,63. Overexpression of SIRT1 has also been shown to decrease plaque burden and improve behavioral phenotypes by deacetylating retinoic acid receptor β, a transcriptional activator of ADAM10. ADAM10 is a component of the α-secretase, which processes APP along an anti-amyloidogenic pathway that decreases formation of toxic Aβ42 species59. An independent study has also shown that SIRT1 expression may decrease levels of ROCK1, a serine/threonine Rho kinase previously shown to regulate Aβ metabolism, and this effect also promotes the non-amyloidogenic α-secretase pathway14,58. The SIRT1 agonist resveratrol was also found to enhance proteasome-mediated clearance of Aβ44 and reduce the plaque burden in the brains of transgenic mice overexpressing APP45,46. Experiments using mixed cortical culture models have also shown that SIRT1 acts to reduce Aβ-stimulated NF-κB signaling in microglia57.