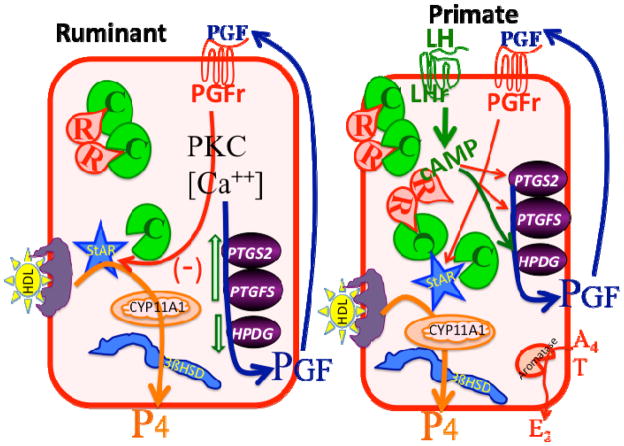
Figure 4.
Postulated model for regulation of large luteal cells of ruminants (left) and primates (right). In the ruminant large luteal cell, there is excess catalytic subunit for PKA (C in green) and this can phosphorylate and activate StAR (steroidogenic acute regulatory protein) as well as other proteins. This produces very high constitutive progesterone (P4) production in the ruminant large luteal cell due to high amounts of cholesterol substrate, in the form of HDL, and high enzymatic activity for CYP11A1 (also known as P450 cholesterol side chain cleavage enzyme) and 3β-hydroxysteroid-dehydrogenase enzyme (3βHSD). Inhibitory pathways that are activated by Prostaglandin F2a (PGF) binding to its receptor (PGFr) are also present, leading to inhibition of StAR, increases in PGF synthesis enzymes (PTGS2 and PTGFS) and inhibition of the PGF degradation enzyme (HPGD). This produces an auto-amplification pathway for PGF with PGF production in the large luteal cell increasing due to PGF action. In the primate large luteal cell, similar pathways for high P4 production are present, however, these pathways are under direct control by LH. Activation of the LH receptor (LHr) by LH increases cAMP, which binds to the regulatory subunit of PKA (R in red), releasing catalytic subunit to phosphorylate StAR and other proteins. In addition, the large luteal cells of primates convert androgens (A4, T) to estrogens (E2). It is postulated that a reduction in LH stimulation would lead to changes in PGF synthesis and degradation enzymes that may increase intraluteal production of PGF and activation of inhibitory pathways by PGF.