Abstract
Purpose
Based on basic research findings an increase in chemokines and cytokines (CXCL-1 and 10, nerve growth factor and interleukin-6) is considered responsible for inflammation and afferent sensitization. In this cross-sectional study we tested the hypothesis that select chemokines are increased in the urine of patients with ulcerative and nonulcerative interstitial cystitis/painful bladder syndrome.
Materials and Methods
Midstream urinary specimens were collected from 10 patients with ulcerative and nonulcerative interstitial cystitis/painful bladder syndrome, respectively, and from 10 asymptomatic controls. Urinary levels of 7 cytokines were measured by a human cytokine/chemokine assay. Nerve growth factor was measured by enzyme-linked immunosorbent assay.
Results
Urinary levels of most chemokines/cytokines were tenfold to 100-fold lower in asymptomatic controls vs patients with ulcerative and nonulcerative interstitial cystitis/painful bladder syndrome. Univariate comparison of 8 tested proteins in the ulcerative vs nonulcerative groups revealed a significant fivefold to twentyfold increase in CXCL-10 and 1, interleukin-6 and nerve growth factor (ANOVA p <0.001).
Conclusions
Differential expression of chemokines in ulcerative and nonulcerative subtypes of interstitial cystitis/painful bladder syndrome suggests differences in paracrine signaling between the 2 entities.
Keywords: urinary bladder, cystitis, interstitial, chemokines, cytokines, ulcer
Interstitial cystitis/PBS is a chronic, painful bladder disease characterized by pelvic pain and irritative voiding symptoms. The subjective perception of the bladder as the source of pain is a necessary criterion for many definitions of IC/PBS. Symptoms associated with IC/PBS can also overlap with those of conditions such as endometriosis, recurrent urinary tract infections, chronic pelvic pain, overactive bladder and vulvodynia.1 All of these features combine to make IC/PBS a difficult disease to diagnose and treat.
Objective disease specific information on patients is only available through expensive, invasive procedures such as cystoscopy and tissue biopsy, which can also categorize IC/PBS into the ulcerative or nonulcerative subtype.2 To avoid the expense and risk of these procedures the development of noninvasive biomarkers to discriminate ulcerative from nonulcerative IC/PBS is a high priority.
Previous studies highlighted important clinical and histopathological distinctions between the 2 subtypes3,4 and led us to suspect that the 2 subtypes may not be different stages of the same disease. Patients with ulcerative and nonulcerative IC/PBS differ in clinical presentation and in the response to treatment. Those with ulcerative IC/PBS tend to have symptoms for a shorter period, perhaps due to older age at diagnosis. The presumed progression from nonulcerative to ulcerative disease is yet to be documented.5 Patients with nonulcerative IC/PBS also have significantly more comorbid pain syndromes and other conditions, and usually respond better to systemic therapy while only cautery of the ulcers (localized therapy) tends to significantly improve symptoms in those with the ulcerative type.5 The 2 subtypes may manifest from different pathophysiological responses to a similar stressor but it is also possible that the 2 subtypes are uniquely different conditions.
To date gene expression studies of bladder tissue from patients have corroborated the findings of histological studies of tissue biopsies from patients with ulcerative IC/PBS.6,7 Many up-regulated genes were expressed in leukocytes, suggesting that immune and inflammatory responses after leukocyte invasion into the bladder wall is a dominant feature of ulcerative IC.6 Gene expression studies in nonulcerative IC/PBS cases revealed urothelial abnormalities, including tight junction proteins, neurokinin receptors and acid sensing channels.8,9 These widely reported findings support the notion that the disease pattern of IC/PBS may be similar to patterns of immune system, lymphatic and autoimmune diseases.
Chemokine expression is a necessary preceding event for the infiltration and cell adhesion into inflamed tissue.10 This innate feature of chemokines can serve as the basis for their usefulness as biomarkers for the phenotypic characterization of IC/PBS.7 Increased mRNA expression of CXCR3 receptor binding chemokines (CXCL-9 to 11) was previously detected in bladder biopsy studies.7 We previously reported an increase in select cytokines and chemokines in the urine of patients with IC/PBS.11
Based on key differences already noted between the subtypes and our clinical observations of patients with ulcerative IC/PBS we hypothesized that identifying urinary biomarkers associated with each may provide new insight into the subtypes as well as the IC/PBS condition overall. Identifying condition specific biomarker panels also meets an ongoing need for simple, accurate, noninvasive methods to detect the disease.
In the current study we investigated whether a multimarker panel of 2 or 3 chemokines in urine could noninvasively differentiate ulcerative from nonulcerative IC/PBS.
MATERIALS AND METHODS
Midstream urinary specimens were collected after receiving informed consent from 10 patients with ulcerative IC/PBS, 10 with nonulcerative IC/PBS and 10 gender matched, asymptomatic outpatient controls with no history of IC, recurrent urinary tract infection, bladder cancer, kidney disease or incontinence (see table). The protocol for the cross-sectional prospective study was approved by the institutional review board.
Table 1.
Table Patient clinical characteristics and chemokine values
| Control | Ulcerative IC/PBS | Nonulcerative IC/PBS | |
|---|---|---|---|
| Median ICSI problem index score | 0.2 | 27.5 | 26.5 |
| Ulcer on cystoscopy | Not applicable | Yes | No |
| Pain | No | Yes | Yes |
| Mean ± SEM chemokine (pg/ml): | |||
| CXCL-1 | 3.54 ± 1.49 | 170 ± 60.55* | 12.55 ± 3.38 |
| CXCL-10 | 41.81 ± 20.13 | 684.7 ± 68.10* | 123.3 ± 45.35 |
| NGF | 2.34 ± 1.3 | 60.82 ± 25.38* | 3.5 ± 1.6 |
| IL-6 | 0.75 ± 0.71 | 16.06 ± 6.99* | 2.42 ± 1.94 |
| MCP-1 | 409.97 ± 162.25 | 672.7 ± 93.75 | 527.97 ± 113.96 |
| RANTES | 4.53 ± 1.72 | 66.34 ± 21.42 | 17.88 ± 3.52 |
| IL-1ra | 349.77 ± 85.23 | 621.96 ± 230.36 | 191.39 ± 94.38 |
| VEGF | 63.67 ± 14.38 | 42.47 ± 14.3 | 22.39 ± 10.67 |
Kruskal-Wallis nonparametric ANOVA with Dunn test 2-tailed p <0.05.
Ulcer in the ulcerative group and its absence in the nonulcerative group of patients with IC/PBS was confirmed by cystoscopy at urine collection. The diagnosis of IC/PBS was based on National Institute of Diabetes and Digestive and Kidney Diseases criteria.1 Other conditions commonly confused with IC/PBS were ruled out. No patient had hydrodistention at least 12 weeks in duration or urinary tract infection when screened before urine collection.
Median age was 61.5 years in the ulcerative IC/PBS group, 48.5 years in the nonulcerative group and 46 years in the control group. Gender distribution was 3 males in the control group, 2 in the ulcerative group and 1 in the nonulcer group. Controls included adults recruited from the community who denied recurrent urinary tract infections, kidney or bladder stones, bladder cancer, kidney disease, urinary incontinence, or IC/PBS diagnosis or symptoms.
All participants completed the validated ICSI problem index at urine collection to assess symptoms of bladder associated pain, abnormal urinary urgency and frequency. Fresh voided urine was immediately centrifuged for 10 minutes at 2,000 rpm at room temperature to remove debris, aliquoted and stored at −80C before chemokine analysis.
Chemokine Assays
On the day of analysis frozen urinary samples were quickly thawed. Seven chemokines/cytokines in urine were measured with the Milliplex® Human Cytokine/Chemokine immunoassay by a previously described procedure.12 We used an automated Bio-Plex® Luminex® 200 IS System immunoassay analyzer, a continuous, random access instrument that performs automated chemiluminescent immunoassays. We assayed chemokines using commercially available microspheres (Millipore®) according to manufacturer instructions.
Microspheres of defined spectral properties conjugated to antibodies directed against CXCL-1 and 10, IL-6, soluble IL-1ra, MCP-1, RANTES and VEGF were pipetted into 96-well plates. Approximately 2,500 antibody conjugated microspheres per chemokine were added to each well. The protocol for these assays involves mixing 50 μl undiluted urine with 25 μl antibody coated microspheres, overnight incubation in the dark at 4C, washing twice with wash buffer, adding detection antibody, adding 25 μl streptavidin-phycoerythrin solution in the well to form a capture sandwich immunoassay by incubation in the dark for 30 minutes at room temperature, washing twice and then adding 150 μl sheath fluid. Microspheres were aspirated through the flow cell of a dual laser Luminex 200 instrument.
Median fluorescence intensity of microspheres specific for each chemokine was recorded for each well to calculate the chemokine concentration by 5-point logistic regression. Urinary NGF was measured by ELISA using a kit (Promega™) according to manufacturer instructions.
Statistical Analysis
Statistical differences in median chemokine levels between the groups were determined by Kruskal-Wallis non-parametric ANOVA, followed by the Dunn test for multi-group comparisons. The Mann-Whitney U test was used for comparisons of 2 groups. Tests assessed significant differences between groups at 2-tailed p <0.05. Data are shown as the mean ± SEM.
RESULTS
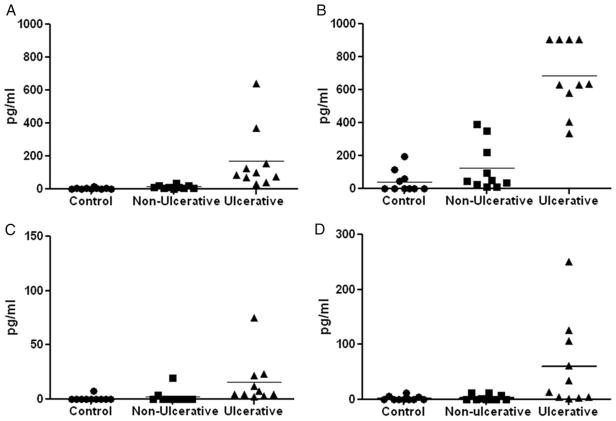
Urine was collected from patients with IC/PBS just before cystoscopy with hydrodistention to evaluate for ulcerative or nonulcerative cystitis. Figure 1 shows representative images from 2 patients per group. Analysis of urine collected from a single void revealed that levels of 5 of the 8 tested chemokines/cytokines in urine were tenfold to 100-fold lower in asymptomatic controls than in patients with IC/PBS. Univariate comparison of 8 tested proteins in the ulcerative and nonulcerative groups revealed a fivefold to twentyfold increase in urinary CXCL-1 and 10, NGF and IL-6 in the former group (2-tailed p <0.001, fig. 2). The distribution of all urinary cytokines tested passed the Kolmogorov-Smirnov test for normality.
Figure 1.
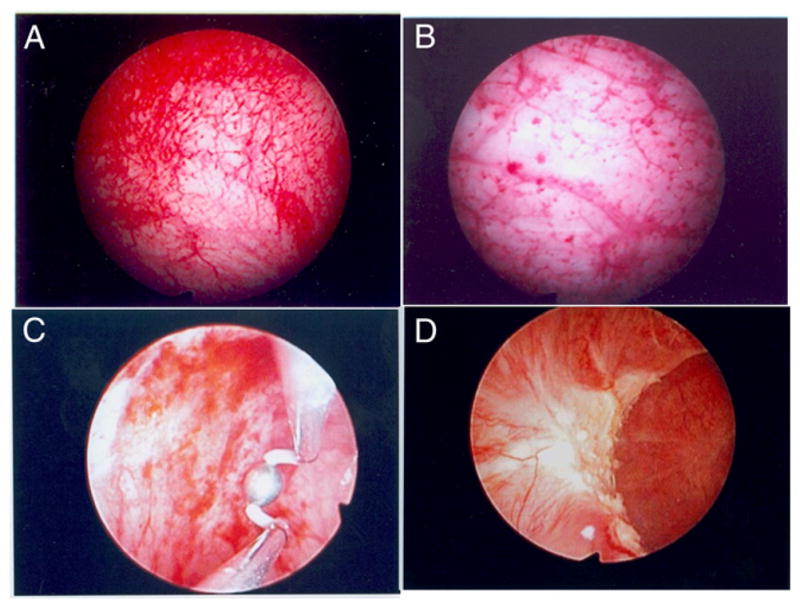
Representative cystoscopic images from patients with nonulcerative (A and B) and ulcerative (C and D) IC/PBS. Urine collected before cystoscopy was used to confirm ulcer, characterized by mucosal bleeding, and inflamed tissue in ulcerative group, and absent ulcer in nonulcerative group. Glomerulations (pinpoint petechial hemorrhages) were noted in nonulcerative group.
Figure 2.
Urinary CXCL-1 (A), CXCL-10 (B), IL-6 (C) and NGF (D) were fivefold to twentyfold, significantly increased in patients with ulcerative IC/PBS vs those with nonulcerative IC/PBS and asymptomatic controls (Kruskal-Wallis ANOVA p <0.01). CXCL-1 was significantly increased in nonulcerative IC/PBS cases vs controls (Mann-Whitney U test p <0.01). NGF and IL-6 were below detection limit in controls. Horizontal bars represent group mean.
We found a significant tenfold increase in ulcerative vs nonulcerative cases in CXCL-1 (mean 170 ± 60.55 vs 12.55 ± 3.38 pg/ml) and a fivefold increase in CXCL-10 (684.7 ± 68.10 vs 123.3 ± 45.35 pg/ml) (Kruskal-Wallis ANOVA p <0.001). There was a twentyfold increase in NGF (60.82 ± 25.38 vs 3.5 ± 1.6) and a fivefold increase in IL-6 (16.06 ± 6.99 vs 2.42 ± 1.94 pg/ml). NGF was below the range of detection in 7 of 10 controls and 7 of 10 nonulcerative cases. IL-6 was below the range of detection in 9 controls and 6 nonulcerative cases.
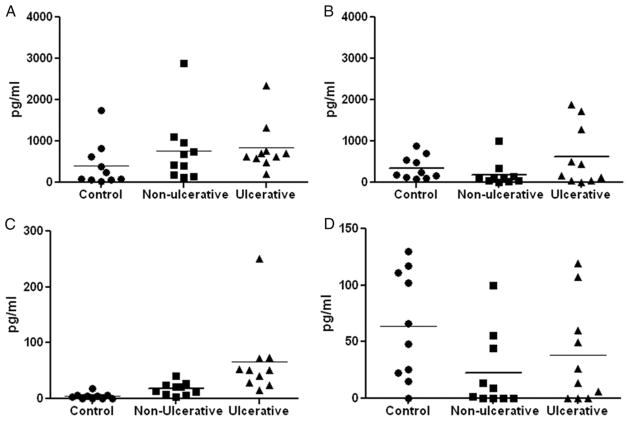
Urinary levels of the remaining proteins, namely MCP-1, sIL-1Ra, RANTES and VEGF, were not significantly increased in the ulcerative IC/PBS group relative to the other groups (see table and fig. 3). Even among these 4 chemokines MCP-1 and IL-1ra were quantitatively higher than RANTES and VEGF. Control levels were also high, which may explain the lack of a significant difference. Two-group comparison of nonulcerative IC/PBS cases and controls revealed a significant increase in CXCL-1 and RANTES in the nonulcerative IC/PBS group (Mann-Whitney U test p <0.01). CXCL-10, sIL-1Ra and MCP-1 levels trending toward significance. The high MCP-1, sIL-1Ra and VEGF levels in the control group agreed with their established role of paracrine messengers under healthy conditions.13
Figure 3.
Urinary MCP-1 (A), IL-1ra (B), RANTES (C) and VEGF (D) in patients with ulcerative IC/PBS were not significantly higher than in those with nonulcerative IC/PBS and asymptomatic controls (Kruskal-Wallis ANOVA p >0.05). RANTES was significantly increased in nonulcerative IC/PBS cases vs controls (Mann-Whitney U test p <0.01). Horizontal bars represent group mean.
Differences in the IC/PBS subtypes were reflected in the patient urinary chemokine profiles. These results lend support to our hypothesis that an increase in chemokines is linked to inflammatory cells, of which the role was previously confirmed in IC/PBS cases.2
DISCUSSION
The urinary proteome receives contributions from filtered plasma proteome and from approximately 20% to 25% of all cellular proteins secreted from the kidney and bladder.14 The quantity of some of these secreted proteins is usually low to merit classification as a biomarker for upper or lower urinary tract disease. In contrast, the level of chemokines secreted in urine from the bladder or kidney is sufficiently high to be detectable by standard laboratory assays. The ability of chemokines to contribute to inflammatory induced changes by inducing chemotaxis, and the activation of mast cells and monocytes in rheumatoid arthritis and Crohn disease was responsible for their qualification as biomarkers.15
Chemokines belong to a family of small secreted glycoproteins with a molecular weight of 7 to 10 kDa and with more than 50 ligands.15 Chemokines are subdivided into 2 major families (CXC and CC) based on the position and number of conserved cysteines, and by the presence of intervening amino acid(s) between the first 2 conserved cysteine residues. The CC class of chemokines, which is distinguished by an adjacent position of the first 2 cysteines, eg MCP-1/CCL2 or RANTES/CCL5, causes chemotactic migration of monocytes, eosinophils, basophils, lymphocytes and mast cells in general but does not act on neutrophils.16 CXC chemokines can be divided into members that contain the ELR motif in the primary structure motif, such as CXCL-1. They are chemoattractants for neutrophils and potent promoters of angiogenesis. In contrast, members that are inducible by interferons, such as CXCL-10, lack the ELR motif (ELR−) and are potent inhibitors of angiogenesis and chemoattractants for mononuclear cells.17
Basic research has revealed that urothelial lesions associated with ulcers result from the infiltration of mononuclear cells and neovascularization (angiogenesis), which is facilitated by growth factors and chemokines.18 Attracting neutrophils and mononuclear cells to the bladder ulcer site requires close interplay of the inflammatory signals presented via chemokines and 7-transmembrane spanning G protein-coupled receptors present on the glycosaminoglycans linked to endothelial cell layers.15 Angiogenesis is a key component of ulcerative inflammation. The disparate impact on angiogenesis of different chemokines measured in urine warrants further investigation.
The chemokines and cytokines that we measured in urine are produced by detrusor smooth muscles.16,19 CXCL-10 is produced as a result of the cascading effect induced by interferon-γ on fibro-blasts, epithelia, monocytes and T cells.20 It is involved in afferent sensitization,21 which may be involved in the mediation of painful bladder symptoms. Urinary CXCL-10 measured in patients with ulcerative IC/PBS was several fold higher than previously reported in the serum of patients with IC/PBS.22 This further corroborates bladder tissue as the primary source of CXCL-10. Higher urinary CXCL-10 also agrees with the higher levels of its transcript detected in bladder tissue.7
As stated earlier, CXCL-10 is a potent inhibitor of angiogenesis17 while VEGF has a key stimulatory role in angiogenesis.23,24 These factors function in autocrine/paracrine pathways to maintain homeostasis, which has been noted in various tissues, including the lung and breast.23,24 VEGF also has an established role as a paracrine messenger in healthy conditions to maintain vascular homeostasis, including the maintenance and function of mature vascular beds.23,24 Presumably this also happens in the bladder. The increase in VEGF noted in controls may be explained by an offsetting homeostatic response to the increase in CXCL-10 (fig. 2, B and C).
The usefulness of CXCL-10 as a reliable drug target for IC/PBS was supported in a preclinical study, in which its blockade by a specific antibody ameliorated chemically induced cystitis.22 Pain is a shared symptom of ulcerative and nonulcerative IC/PBS. Based on our findings the chemokines that mediate pain may be different in ulcerative and nonulcerative IC/PBS cases. CXCL-10 seems to predominate in mediating the pain symptom of ulcerative IC/PBS while MCP-1 and other chemokines may mediate the pain symptom of nonulcerative IC/PBS by influencing nociceptive fibers.25
Study limitations include our few patients and urinary measurement made at only 1 time point. The increased urinary levels in patients with ulcerative IC/PBS reflect the ongoing bladder inflammation noted at the bladder ulcer site. Passive secretion of chemokines from bladder to stored urine is independent of the urinary flow rate downstream of the kidney. Thus, no additional benefit would have accrued from adjusting chemokine levels to creatinine. Adjusting protein to creatinine did not alter the outcome in previous studies in which bladder epithelium derived growth factors were measured in the urine of patients with IC/PBS and controls.26 Similar results were obtained in series of bladder malignancy and a urinary biomarker.27
Higher NGF levels in the ulcerative IC/PBS group agree with previous reports on this topic.28 The urinary levels of IL-6 that we noted in patients with IC/PBS agreed with reports from other groups.29 IL-6 levels were comparatively lower than levels of other chemokines (CXCL-1 and 10) (fig. 2). Basic research revealed that chemokines and cytokines (CXCL-1 and 10, NGF and IL-6) have key responsibility for inflammation and afferent sensitization.
The Luminex xMAP technology used in our series applies a sandwich format, as used in traditional ELISA exploiting antibody specificity.30 This provides higher specificity for chemokines and minimizes cross-reactivity with other urinary proteins. Multiplexing analytes in solution phase through flow cytometry and equilibrium in antibody-antigen reactions is attained more quickly in the solution phase to increase assay efficiency.
Biomarker proteins detected in urine and other biological fluids are generally assumed to reflect the authentic biochemistry of the disease site with which they remain in intimate contact. They are not generally as subject to imperfect human perceptions or to opinions as the ICSI score. Identifying urinary biomarkers by proteome-wide analysis may provide an unbiased, noninvasive, expeditious discrimination of IC/PBS into ulcerative and nonulcerative groups. In conclusion, differential expression of chemokines in association with ulcerative and non-ulcerative IC/PBS suggests differences in paracrine signaling between the 2 entities.
Acknowledgments
Supported by the Ministrelli Program for Urology Research and Education.
Abbreviations and Acronyms
- CXCL
CXC chemokine ligand
- ELR
Glu-Leu-Arg
- IC
interstitial cystitis
- ICSI
IC Symptom Index
- IL
interleukin
- IL-1ra
IL-1 receptor antagonist
- MCP-1
monocyte chemoattractant protein-1
- NGF
nerve growth factor
- PBS
painful bladder syndrome
- RANTES
regulated on activation, normal T-cell expressed and secreted
- sIL-1Ra
soluble IL-1ra
- VEGF
vascular endothelial growth factor
Footnotes
Study received institutional review board approval.
References
- 1.Hanno PM, Landis JR, Matthews-Cook Y, et al. The diagnosis of interstitial cystitis revisited: lessons learned from the National Institutes of Health Interstitial Cystitis Database study. J Urol. 1999;161:553. doi: 10.1016/s0022-5347(01)61948-7. [DOI] [PubMed] [Google Scholar]
- 2.Erickson DR, Tomaszewski JE, Kunselman AR, et al. Do the National Institute of Diabetes and Digestive and Kidney Diseases cystoscopic criteria associate with other clinical and objective features of interstitial cystitis? J Urol. 2005;173:93. doi: 10.1097/01.ju.0000146466.71311.ab. [DOI] [PubMed] [Google Scholar]
- 3.Koziol JA, Adams HP, Frutos A. Discrimination between the ulcerous and the nonulcerous forms of interstitial cystitis by noninvasive findings. J Urol. 1996;155:87. [PubMed] [Google Scholar]
- 4.Peeker R, Fall M. Toward a precise definition of interstitial cystitis: further evidence of differences in classic and nonulcer disease. J Urol. 2002;167:2470. [PubMed] [Google Scholar]
- 5.Peters KM, Killinger KA, Mounayer MH, et al. Are ulcerative and nonulcerative interstitial cystitis/painful bladder syndrome 2 distinct diseases? A study of coexisting conditions. Urology. 2011;78:301. doi: 10.1016/j.urology.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 6.Gamper M, Viereck V, Geissbuhler V, et al. Gene expression profile of bladder tissue of patients with ulcerative interstitial cystitis. BMC Genomics. 2009;10:199. doi: 10.1186/1471-2164-10-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogawa T, Homma T, Igawa Y, et al. CXCR3 binding chemokine and TNFSF14 over expression in bladder urothelium of patients with ulcerative interstitial cystitis. J Urol. 2010;183:1206. doi: 10.1016/j.juro.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez Freire V, Burkhard FC, Kessler TM, et al. MicroRNAs may mediate the down-regulation of neurokinin-1 receptor in chronic bladder pain syndrome. Am J Pathol. 2010;176:288. doi: 10.2353/ajpath.2010.090552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sánchez-Freire V, Blanchard MG, Burkhard FC, et al. Acid-sensing channels in human bladder: expression, function and alterations during bladder pain syndrome. J Urol. 2011;186:1509. doi: 10.1016/j.juro.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 10.Perez de Lema G, Maier H, Nieto E, et al. Chemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritis. J Am Soc Nephrol. 2001;12:1369. doi: 10.1681/ASN.V1271369. [DOI] [PubMed] [Google Scholar]
- 11.Tyagi P, Nikolavsky D, Vodovotz Y, et al. Urine levels of selected chemokines positively correlate with lower bladder capacity and psychometric scores in IC/PBS patients. J Urol. 2009;181:21. [Google Scholar]
- 12.Tyagi P, Barclay D, Zamora R, et al. Urine cytokines suggest an inflammatory response in the overactive bladder: a pilot study. Int Urol Nephrol. 2010;42:629. doi: 10.1007/s11255-009-9647-5. [DOI] [PubMed] [Google Scholar]
- 13.Sadeghi M, Daniel V, Naujokat C, et al. Strikingly higher interleukin (IL)-1alpha, IL-1beta and soluble interleukin-1 receptor antagonist (sIL-1RA) but similar IL-2, sIL-2R, IL-3, IL-4, IL-6, sIL-6R, IL-10, tumour necrosis factor (TNF)-alpha, transforming growth factor (TGF)-beta and interferon IFN-gamma urine levels in healthy females compared to healthy males: protection against urinary tract injury? Clin Exp Immunol. 2005;142:312. doi: 10.1111/j.1365-2249.2005.02924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diamandis EP. How are we going to discover new cancer biomarkers? A proteomic approach for bladder cancer. Clin Chem. 2004;50:793. doi: 10.1373/clinchem.2004.032177. [DOI] [PubMed] [Google Scholar]
- 15.Mortier A, Van Damme J, Proost P. Regulation of chemokine activity by posttranslational modification. Pharmacol Ther. 2008;120:197. doi: 10.1016/j.pharmthera.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Bouchelouche K, Alvarez S, Andersen L, et al. Monocyte chemoattractant protein-1 production by human detrusor smooth muscle cells. J Urol. 2004;171:462. doi: 10.1097/01.ju.0000090192.36436.d5. [DOI] [PubMed] [Google Scholar]
- 17.Addison CL, Daniel TO, Burdick MD, et al. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol. 2000;165:5269. doi: 10.4049/jimmunol.165.9.5269. [DOI] [PubMed] [Google Scholar]
- 18.Ueda T, Tamaki M, Ogawa O, et al. Over expression of platelet-derived endothelial cell growth factor/thymidine phosphorylase in patients with interstitial cystitis and bladder carcinoma. J Urol. 2002;167:347. [PubMed] [Google Scholar]
- 19.Bouchelouche K, Alvarez S, Horn T, et al. Human detrusor smooth muscle cells release interleukin-6, interleukin-8, and RANTES in response to proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha. Urology. 2006;67:214. doi: 10.1016/j.urology.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 20.Antoniou KM, Tzanakis N, Tzortzaki EG, et al. Different angiogenic CXC chemokine levels in bronchoalveolar lavage fluid after interferon gamma-1b therapy in idiopathic pulmonary fibrosis patients. Pulm Pharmacol Ther. 2008;21:840. doi: 10.1016/j.pupt.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Bhangoo S, Ren D, Miller RJ, et al. Delayed functional expression of neuronal chemokine receptors following focal nerve demyelination in the rat: a mechanism for the development of chronic sensitization of peripheral nociceptors. Mol Pain. 2007;3:38. doi: 10.1186/1744-8069-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakthivel SK, Singh UP, Singh S, et al. CXCL10 blockade protects mice from cyclophosphamide-induced cystitis. J Immune Based Ther Vaccines. 2008;6:6. doi: 10.1186/1476-8518-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lazarus A, Keshet E. Vascular endothelial growth factor and vascular homeostasis. Proc Am Thorac Soc. 2011;8:508. doi: 10.1513/pats.201102-021MW. [DOI] [PubMed] [Google Scholar]
- 24.Dabrosin C. Variability of vascular endothelial growth factor in normal human breast tissue in vivo during the menstrual cycle. J Clin Endocrinol Metab. 2003;88:2695. doi: 10.1210/jc.2002-021584. [DOI] [PubMed] [Google Scholar]
- 25.Jung H, Toth PT, White FA, et al. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J Neurochem. 2008;104:254. doi: 10.1111/j.1471-4159.2007.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keay S, Zhang CO, Kagen DI, et al. Concentrations of specific epithelial growth factors in the urine of interstitial cystitis patients and controls. J Urol. 1997;158:1983. doi: 10.1016/s0022-5347(01)64198-3. [DOI] [PubMed] [Google Scholar]
- 27.Shariat SF, Matsumoto K, Casella R, et al. Urinary levels of soluble ecadherin in the detection of transitional cell carcinoma of the urinary bladder. Eur Urol. 2005;48:69. doi: 10.1016/j.eururo.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Liu HT, Tyagi P, Chancellor MB, et al. Urinary nerve growth factor level is increased in patients with interstitial cystitis/bladder pain syndrome and decreased in responders to treatment. BJU Int. 2009;104:1476. doi: 10.1111/j.1464-410X.2009.08675.x. [DOI] [PubMed] [Google Scholar]
- 29.Lamale LM, Lutgendorf SK, Zimmerman MB, et al. Interleukin-6, histamine, and methylhistamine as diagnostic markers for interstitial cystitis. Urology. 2006;68:702. doi: 10.1016/j.urology.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 30.Kofoed K, Schneider UV, Scheel T, et al. Development and validation of a multiplex add-on assay for sepsis biomarkers using xMAP technology. Clin Chem. 2006;52:1284. doi: 10.1373/clinchem.2006.067595. [DOI] [PubMed] [Google Scholar]