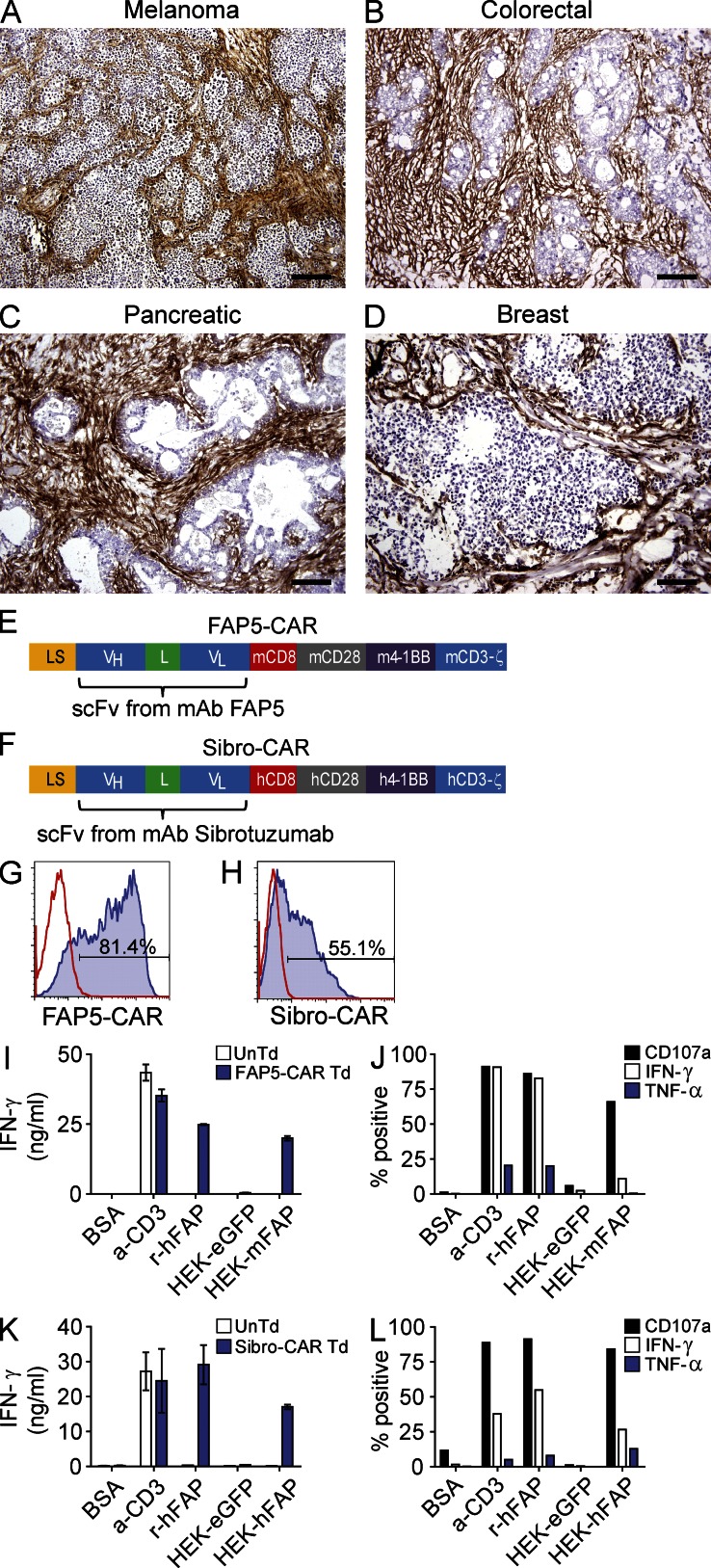
Figure 1.
IHC staining for FAP in various human tumors, and design and in vitro activity of FAP-reactive CARs. Representative IHC staining for FAP in human melanoma (A), colorectal (B), pancreatic (C), and breast (D) adenocarcinomas. Isotype stains were negative (not depicted). Bars: 400 µm (A); 200 µm (B–D). Schematic of the FAP-reactive CAR constructs FAP5-CAR (E) and Sibro-CAR (F). LS, GM-CSFR leader sequence; VH and VL, variable heavy and light chains; L, 218 linker; CD8, transmembrane domain; CD28, 4-1BB, and CD3-ζ, intracellular signaling domains; m, murine; h, human. Both constructs were cloned into the MSGV1 retroviral vector. Retrovirus containing FAP5-CAR or Sibro-CAR constructs were generated and used to transduce mouse and human T cells, respectively, and flow cytometry was used to assess transduction efficiency at day 2 after transduction for FAP5-CAR (G) and day 8–10 after transduction for Sibro-CAR (H). Solid line is isotype control and filled histogram is FAP5 or Sibrotuzumab stained. Day 5-stimulated untransduced (UnTd) and FAP5-CAR–transduced (Td) mouse T cells were assessed for reactivity against plate-bound BSA, α-CD3 mAb, and recombinant human FAP (r-huFAP), and against HEK293 cell lines expressing or not expressing FAP. After an overnight stimulation, supernatants were assessed for IFN-γ with an IFN-γ ELISA (I), and cells were further assessed for cell surface CD107a expression, and production of IFN-γ and TNF by ICS (J). For ICS, cells are gated on FAP5-CAR Td cells. Day ∼14-stimulated UnTd or Sibro-CAR Td human T cells were assessed for in vitro reactivity as described for mouse. IFN-γ ELISA (K), and ICS results gated on Sibro-CAR Td T cells (L) are shown. Mean ± SD. All results are representative of at least three independent experiments.