Fig. 1.
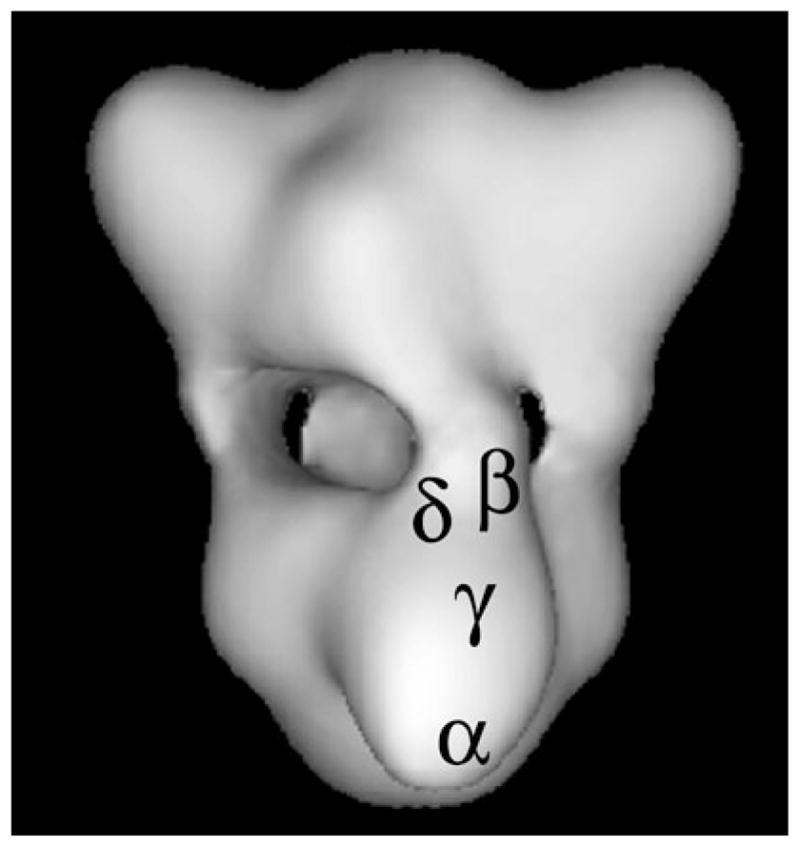
Three-dimensional structure of PhK at 25 Å resolution reconstructed from ~5,000 images of frozen hydrated particles in cryoEM (16). Each lobe is an (αβγδ)2 octamer and four bridges separate the two lobes. The positions of specific regions of the four subunits of a single αβγδ protomer are denoted. The regions of the α, β, and γ subunits represent epitopes recognized by monoclonal antibodies that were localized by immunoEM of negatively stained complexes (14, 15), with those locations then transferred to the cryoEM structure. The δ subunit was directly visualized by scanning transmission EM of PhK in which a fraction of its δ subunits had been exchanged with mutant calmodulin derivatized with Nanogold (17). Its location was also then transferred to the cryoEM model.
