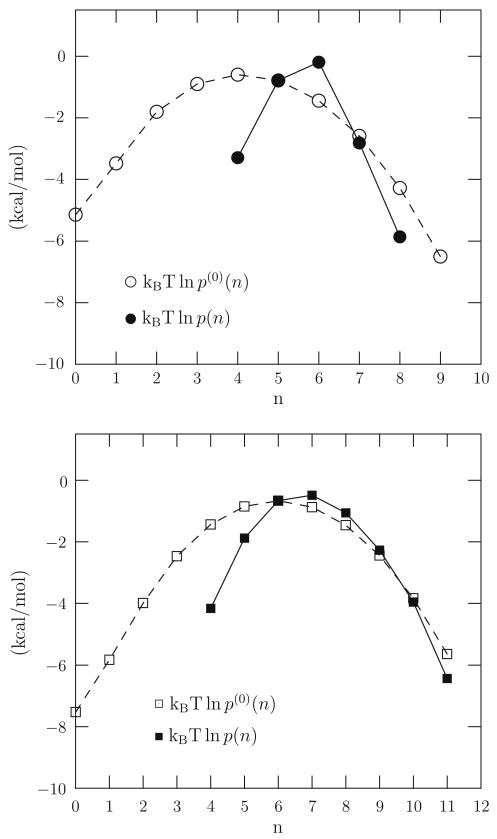
Fig. 2.
Occupancy distributions obtained by molecular dynamics simulation for the cases of Na+(aq) (upper) and K+(aq) (lower). The calculations used the NAMD program [25] and the TIP3P [26] model of water. T = 298 K (Langevin thermostat) and the cubic simulation system contained 306 water molecules for the pure water simulation; the ion-water system had an additional ion. The total number density (counting water molecules and the ion, if present) is 33:33 nm−3. For Na+(aq) the inner shell radius was 3.1 Å, and for K+(aq) it was 3.5 Å. These are the radii of the first minimum in the ion-oxygen radial distribution in liquid water at infinite dilution.