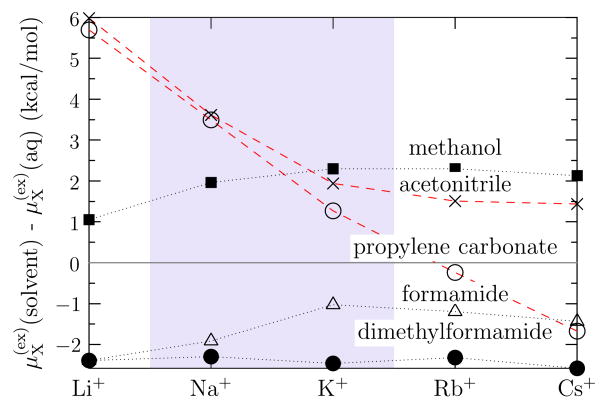
Fig. 5.
Standard free energies of transfer from aqueous solution to dilute solution of the solvent indicated at infinite electrolyte dilution, T = 298.15 K, and standard pressure [45]. Absolute values for these transfer free energies involve extrather-modynamical assumptions [45,46]. Differences of these transfer free energies between different ions do not depend on those extrathermodynamical assumptions. These data give a perspective on the possibility of selectivity that is broader than just comparison of Na+ and K+ (shaded), but do not address biomolecular features that might arise with ion channels.