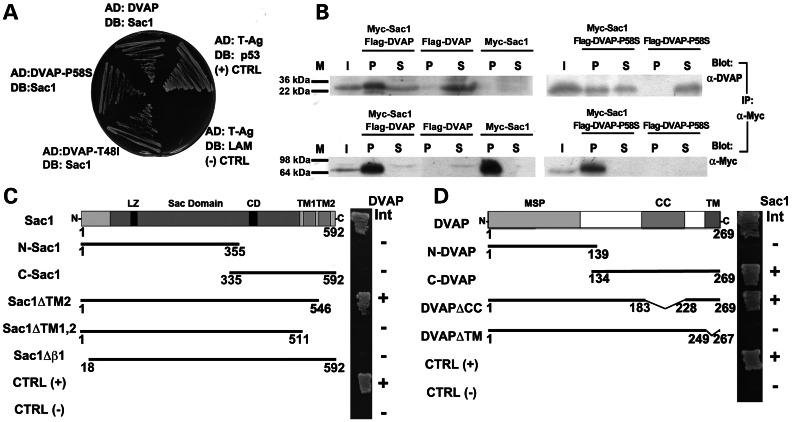
Figure 1.
Sac1 interacts with both DVAP and DVAP-P58S. (A) Sac1 interaction with DVAP and DVAP-P58S by yeast two-hybrid assay. p53/T-Ag interaction is the positive control and the lack of interaction between T-Ag and Lamin (LAM) is used as a negative control. Sac1 interacts with DVAP as well as with DVAP-P58S and DVAP-T48I. AD, Gal4 activation domain. DB, Gal4 DNA-binding domain. (B) Co-immunoprecipitation of Myc-Sac1 and Flag-DVAP or Flag-DVAP-P58S from cell lysates transfected with the indicated plasmids. Immunoprecipitates were collected on anti-Myc Sepharose beads. Input (I), immunoprecipitated complexes indicated as pellet (P) and the unbound fractions indicated as supernatant (S) were analysed by western blots with antibodies to DVAP or Myc. (C) DVAP interaction with Sac1-truncated proteins. LZ, leucine zipper; CD, catalytic domain; TM1,TM2, transmembrane domains 1, 2. On the right column, interactions (Int) are indicated by plus signs and lack of interaction by minus signs. In (D), Sac1 interaction with DVAP-truncated proteins is reported. MSP, major sperm protein; CC, coiled coil; TM, transmembrane domains. Interactions (Int) are indicated by plus signs and lack of interaction is indicated by minus signs. The CC domain (amino acids 184–227) and the TM domain (amino acids 250–266) were deleted from DVAP.