Figure 2. CU pilin subunit structure.
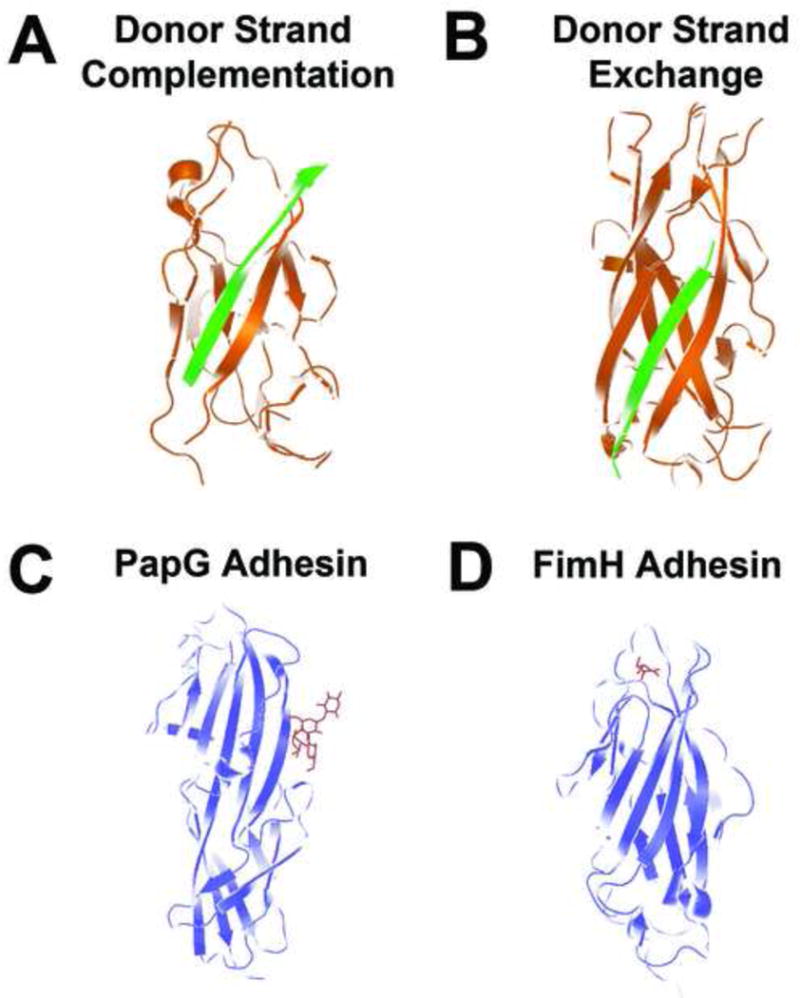
(a) Donor strand complementation. The interactive subunit assembly site created by the incomplete immunoglobulin fold of the PapE (orange) pilin subunit is capped by the G1 strand of the PapD chaperone (green), providing the steric information necessary to complement the Ig-like fold of the subunit and stabilize the subunit prior to pilus assembly (PDB code 1NOL). (b) Donor strand exchange. PapE (orange) pilin structure consists of an incomplete Ig fold, which is completed within the pilus structure by the N-terminal extension of the neighboring pilin subunit, PapK (green), to form a canonical Ig domain in the polymerized pilus fiber (PDB code 1N12). (c) PapG ligand-binding domain (blue) bound to GbO4 (red), which consists of the PapG ligand tetrasaccharide GalNAc beta 1–3Gal alpha 1–4Gal beta 1–4Glc linked to ceramide (PDB code 1J8R). (d) FimH ligand-binding domain interacting with its D-mannose ligand (red) (PDB code 1KLF).
