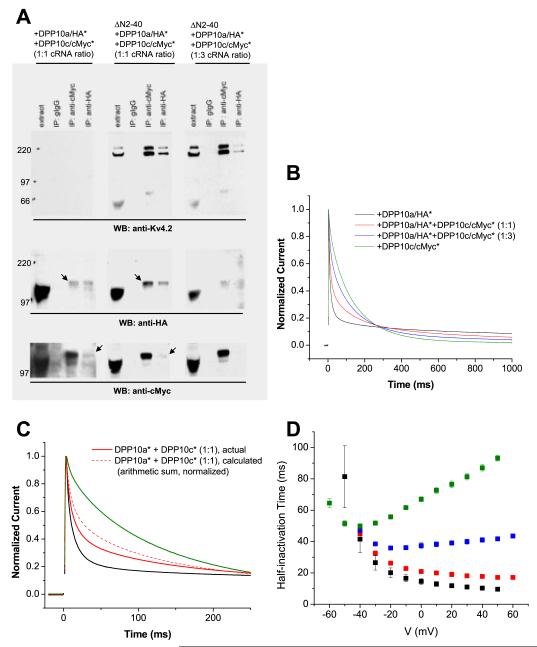
Figure 6. DPP10a-mediated fast inactivation prevails in the presence of DPP10c.
HA and cMyc tags were inserted in an extracellular loop of DPP10a (DPP10a/HA*) and DPP10c (DPP10c/cMyc*), respectively, as to not interfere with the DPP10a N-terminal domain’s function. (A) Co-immunoprecipitation studies show that Kv4.2 does not interfere with DPP10 variants heteromerization. Oocytes extracts of were subjected to immunoprecipitation procedures as described in the experimental methods section. In the absence of Kv4.2, anti-HA antibody immunoprecipitate DPP10c/cMyc* along with DPP10a/HA*, and vice versa, indicating DPP10 variant heteromultimerization (left panels). When introduced, ΔN2-40 proteins binds to both variants and, most importantly, do not interfere with this co-immunoprecipitation of DPP10 variants (middle and right panels). (B) Normalized currents at +50 mV elicited from oocytes co-expressing ΔN2-40 with DPP10a/HA* alone, DPP10c/cMyc* alone, and DPP10a/HA* plus DPP10c/cMyc* at 1:1 and 1:3 cRNA molar ratios. (C) Comparison of ΔN2-40+DPP10a/HA*+DPP10c/cMyc* (1:1) current compared to arithmetic sum of ΔN2-40+DPP10a/HA-int and ΔN2-40/cMyc-int current. The two currents are significantly different, consistent with the formation of different DPP10 variants bound to same channel complexes. (D) Voltage-dependence of half-inactivation times for currents expressed by oocytes injected with ΔN2-40 plus DPP10a/HA* (black symbols), plus DPP10a/HA* and DPP10c/cMyc* at 1:1 cRNA ratio (red symbols), plus DPP10a/HA* and DPP10c/cMyc* at 1:3 cRNA ratio (blue symbols), and plus DPP10c/cMyc* (green symbols).