Figure 3.
Functional Characterization of BICD2 Mutations
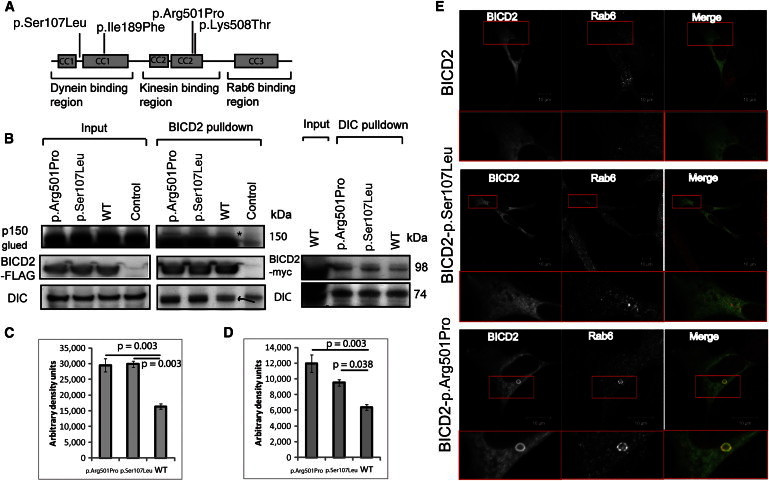
(A) A schematic diagram demonstrating the three BICD2 regions composed of five coiled-coil domains, the four identified alterations, and known interacting proteins.
(B) Coimmunoprecipitation studies of HEK293 cells transfected with BICD2 C terminally tagged with FLAG-Myc show a higher interaction between the p.Arg501Pro and p.Ser107Leu altered proteins and DIC and the p150Glued subunit of dynactin (band shown with ∗). The control represents a nontransfected cell lysate. For the FLAG-BICD2 pulldown, the pulled down DIC is highlighted by an arrow (the upper band is present in the nontransfected control). Reverse immunoprecipitation with DIC antibody confirmed the increased interaction between the p.Arg501Pro and p.Ser107Leu variants and DIC.
(C and D) Optical densitometry for DIC and dynactin p150Glued pulldown for a single immunoprecipitation performed in triplicate. ANOVA p = 0.003 in (C), and ANOVA p = 0.004 in (D). Error bars represent the SEM.
(E) Immunocytochemistry of SH-SY5Y cells transfected with constructs encoding p.Arg501Pro and p.Ser107Leu and stained for Myc and RAB6 show the presence of a BICD2 p.Arg501Pro-RAB6 ring structure.
