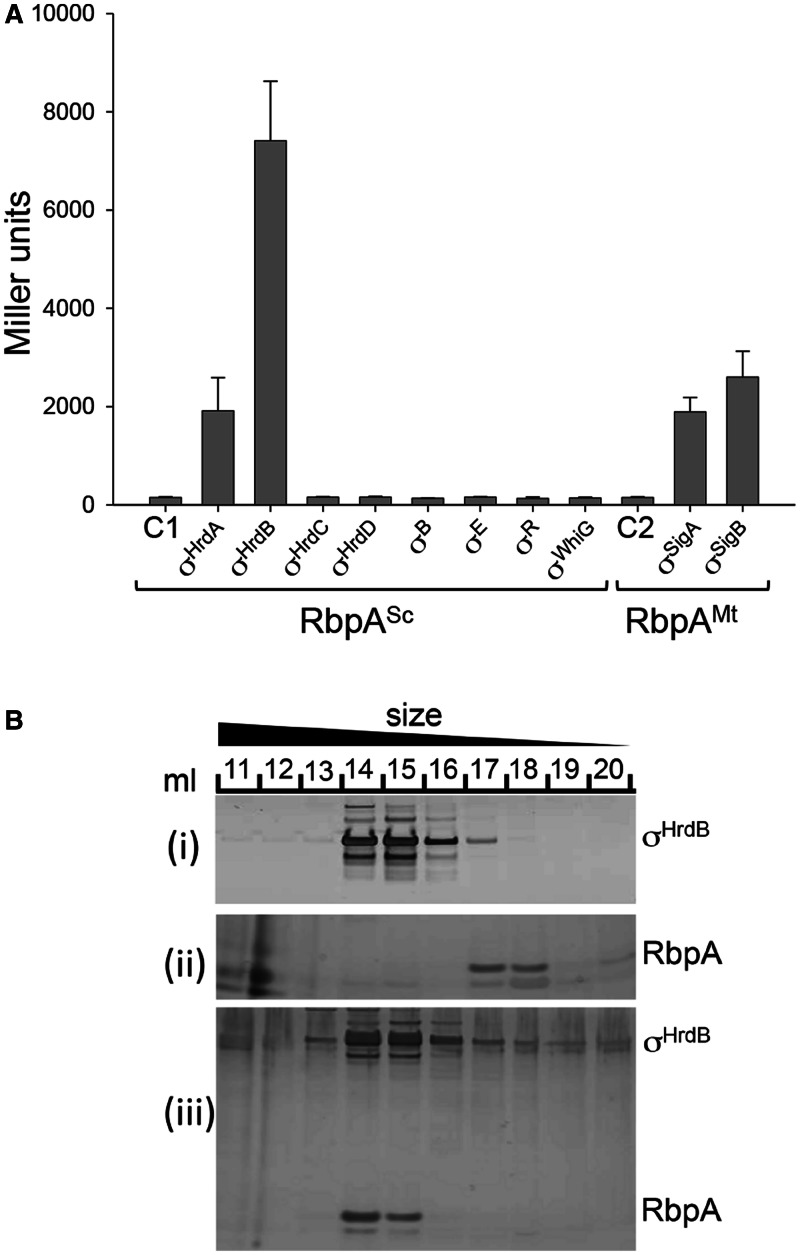
Figure 3.
RbpA binds to the principal and closely related σ factors. (A) BACTH analysis of RbpA from S. coelicolor (RbpASc) and M. tuberculosis (RbpAMt) interactions with σ factors from S. coelicolor (Group 1, σHrdB; Group 2, σHrdA, σHrdC and σHrdD; Group 3, σHrdB and σWhiG; Group 4, σE and σR) and M. tuberculosis (Group 1, σA; Group 2, σB). The rbpA genes were fused to the T18 subunit of B. pertussis adenylate cyclase, whereas the σ factor genes were fused to the T25 subunit. Groups 1 and 2 σ factor hybrid fusions included only σ domains σ2, σ3 and σ4, whereas the remaining σ factor fusions were full length. β-galactosidase assays were performed in triplicate, and standard deviations are indicated. Control strains contained C1, pUT18-rbpASc, pKT25; C2, pUT18-rbpAMt, pKT25. (B) Co-elution of RbpASc and σHrdB during size-exclusion chromatography. (i) σHrdB (5 nmol), (ii) RbpASc (5 nmol) or a mixture of the two (iii) were passed through a Superose 6 10/300 GL size-exclusion column, and 1 ml of fractions (indicated by numbers) were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and silver stained.